A kind of synthesis technique of chiral catalyst
A synthesis process, chiral technology, applied in the field of low-cost and high-efficiency synthesis process, can solve problems such as difficulty in realization, high energy consumption at low temperature control, and strong odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
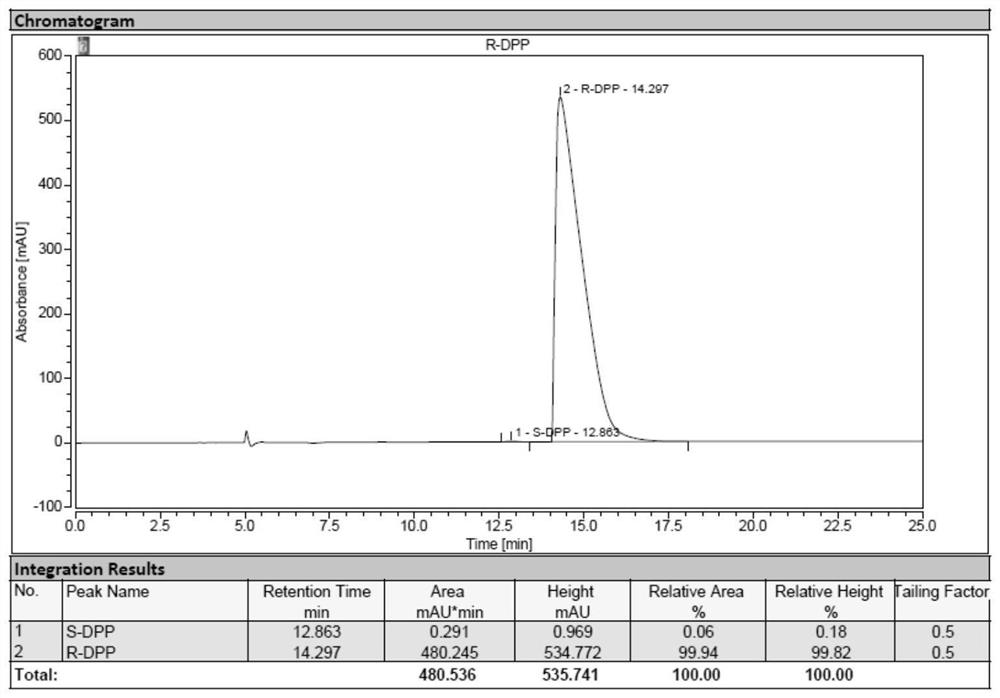
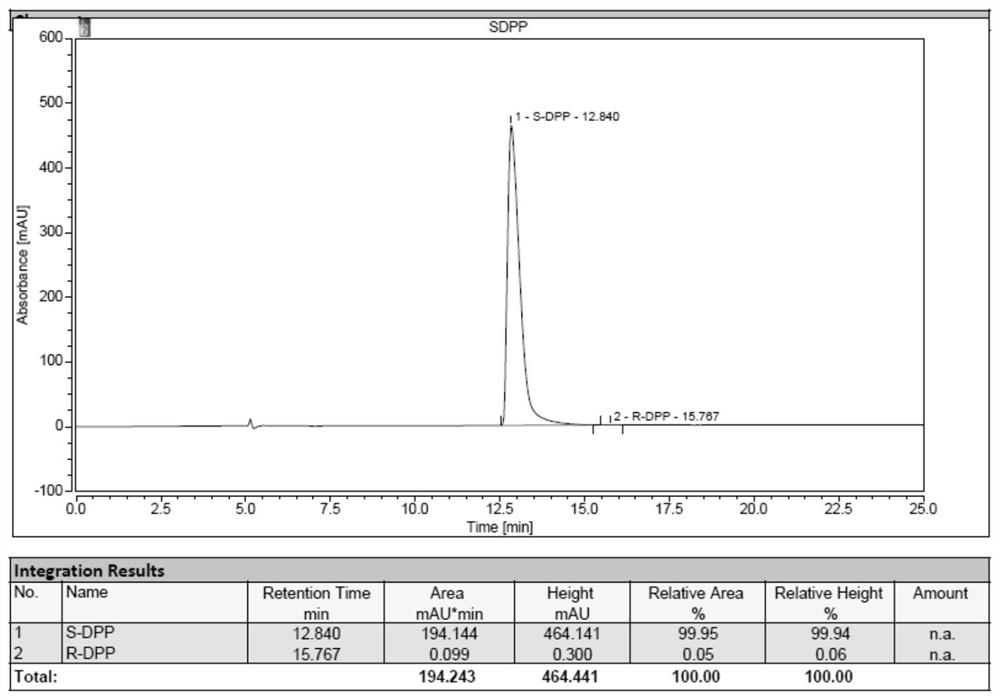
[0077] The industrialized production of embodiment 1 (R)-diphenylprolinol hydrochloride
[0078]
[0079] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. 7.0 kg of concentrated sulfuric acid (mass concentration 98%) is slowly pumped into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of D-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0080] 2. Pump 35.0 kg of dichloromethane into the reactor. Cool down to 15°C, add 14.8 kg of sodium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the re...
Embodiment 2
[0085] The industrialized production of embodiment 2 (R)-diphenylprolinol hydrochloride
[0086] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. Slowly pump 7.0 kg of concentrated sulfuric acid into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of D-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0087] 2. 34.3 kg of tetrahydrofuran was pumped into the reaction kettle. Cool down to 15°C, add 19.2 kg of potassium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the reaction kettle, add dropwi...
Embodiment 3
[0090] The industrialized production of embodiment 3 (S)-diphenylprolinol hydrochloride
[0091]
[0092] 1. A 100-liter reactor was pumped into 22.0 kilograms of anhydrous methanol under nitrogen protection. Control the internal temperature of the reactor below 30°C. Slowly pump 7.0 kg of concentrated sulfuric acid into the reactor. Open the lid of the reaction kettle, and quickly add 8.0 kg of L-proline. After the addition, adjust the inner temperature of the reaction kettle to 25-30° C., and continue stirring for 7 hours. The solvent was distilled off under reduced pressure at 55°C.
[0093] 2. Pump 35.0 kg of dichloromethane into the reactor. Cool down to 15°C, add 14.8 kg of sodium carbonate in batches, keep the temperature below 20°C during the addition, and continue stirring for 30 minutes after the addition. Pump the dichloromethane (11.2 kg) solution of di-tert-butyl dicarbonate (16.1 kg) into the supporting dropping tank of the reaction kettle, add dropwise at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com