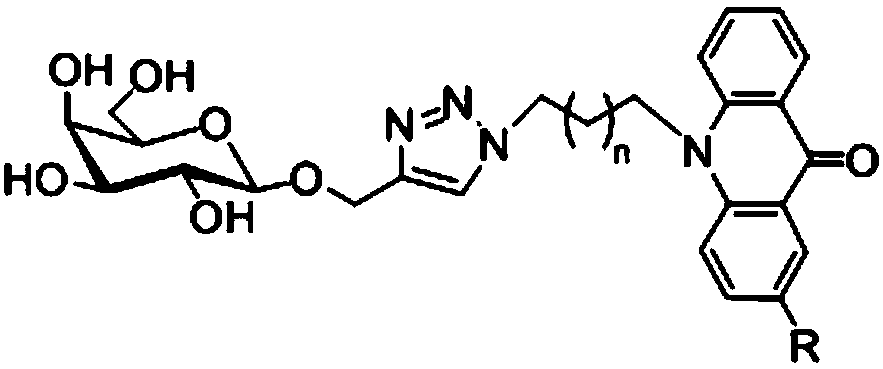
Acridone derivative containing galactose, preparation method and application thereof
A technology of acridone and galactose, applied in the field of acridone derivatives and its preparation, can solve the problem of no acridone derivatives, etc., achieve good biological activity and anticancer activity, less by-products, and less reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
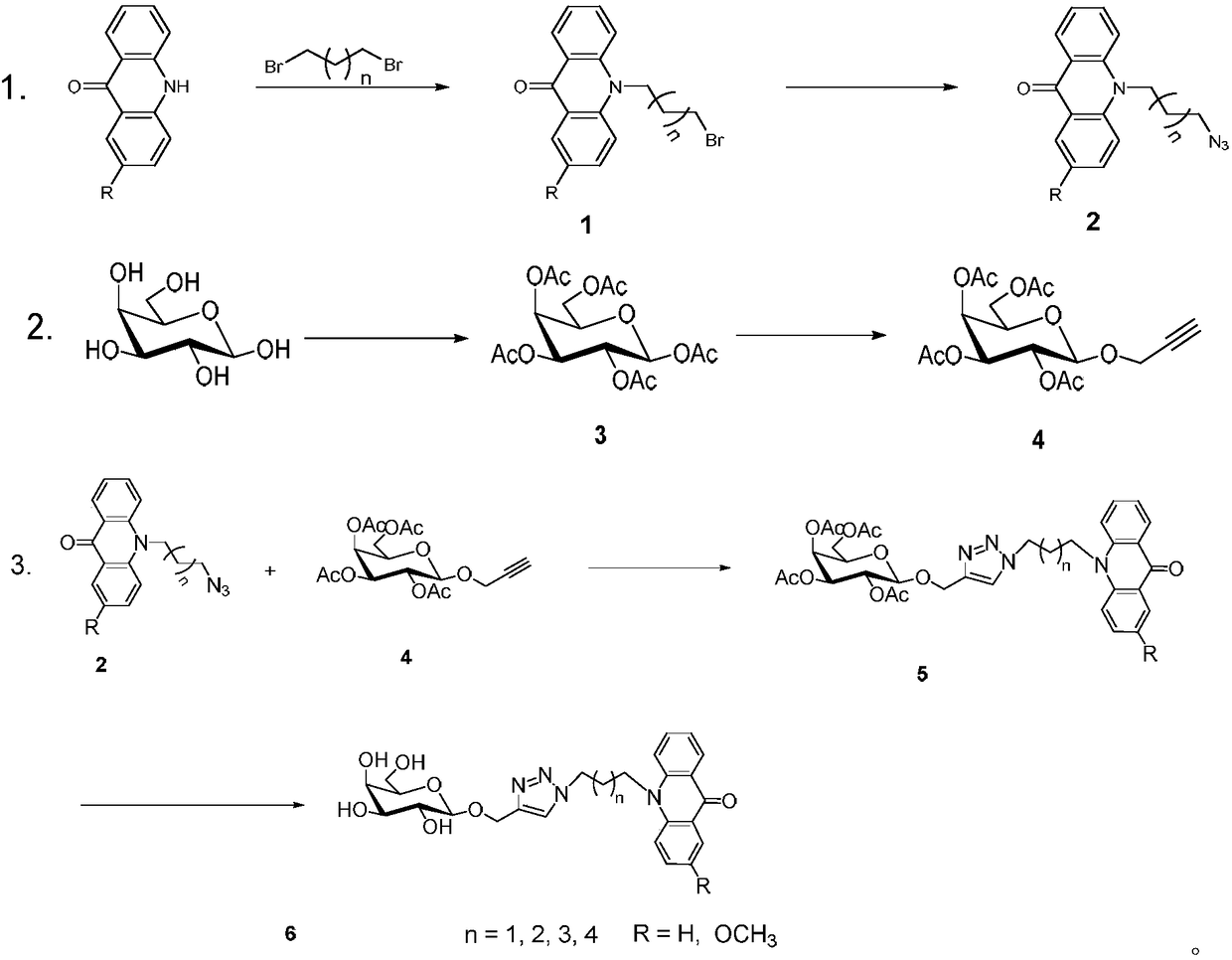
[0023] Take the above compound as an example to specifically describe the synthesis process:
[0024] Step S1: Using DMF as a solvent, put a small amount of 9(10H)-acridone (98wt%) (1g about 5.1mmol) into a round bottom flask, place it on a constant temperature magnetic stirrer, and then add (0.41g about 10.2 mmol) sodium hydride, stirred at room temperature for about 1.5 h, then added dropwise (3.1 mL about 30.6 mmol) of 1.3-dibromopropane to the reaction system, placed in an oil bath at 80° C. for about 24 h, and took it out. Wait until the reaction system is down to room temperature, extract with ethyl acetate, after the TLC spot plate monitors the reaction is complete, dry the organic phase, rotary evaporate and mix the sample, and separate through column chromatography to obtain compound 1 acridine bromide (0.95g about 3mmol, yield 59%).
[0025] 1 HNMR (600MHz, CDCl 3 )δ8.52(d, J=7.8Hz, 2H), 7.68(t, J=7.8Hz, 2H), 7.50(d, J=8.4Hz, 2H), 7.24(t, J=7.4Hz, 2H) ...
Embodiment 2
[0042] Some structures of the compounds obtained according to the above synthesis method:
[0043]
Embodiment 3
[0045] Activity data analysis of preliminary determination of some compounds:
[0046] The anticancer activity of the synthesized galactose-containing acridone derivatives was determined by MTT method. Human gastric cancer cells MGC-803 and human breast cancer cells MCF-7 were respectively inoculated in the corresponding medium containing 10 wt% fetal calf serum, at 37 °C, with a volume fraction of 5% CO 2 and saturated humidity conditions, the logarithmic growth phase cells were inoculated into 96-well culture plates, incubated in the incubator for 24 hours, and replaced with different concentrations of compounds (0, 3, 6, 9, 12, 15, 18 μM / L) complete culture medium. After culturing for 72 hours, add 20 μL of MTT (5 mg / mL), continue to cultivate for 4 hours, then slowly absorb the supernatant, add 150 μL of DMSO, shake and shake for 10 minutes to fully dissolve the crystals, and then use a microplate reader to detect the absorbance.
[0047] Preliminary biological activity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com