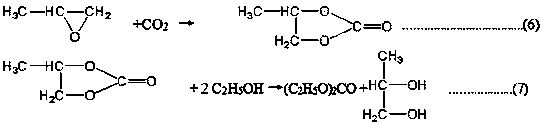Production process of high-purity diethyl carbonate through direct catalysis of solid base catalyst
A solid base catalyst, the technology of diethyl carbonate, applied in the purification/separation of carbonate/haloformate, the preparation of organic carbonate, the production of bulk chemicals, etc., can solve the waste of raw materials, low solubility, Problems such as poor catalyst stability, to achieve the effects of high product selectivity and yield, simple process flow, and short synthesis path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
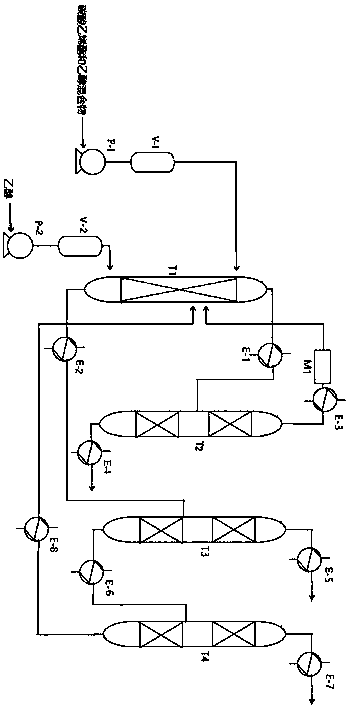
[0073] Ethylene carbonate and excess ethanol are used as raw materials to react to produce diethyl carbonate and ethylene glycol at a ratio of 1:1, and the distilled ethanol is recycled.
[0074] The catalyst is an alkaline composite material 15%BaO-5%MgO-3%La 2 o 3 / Cs-meso-EMT, the cross-sectional area ratio of the initial distillation section and the side line section is 1:1.
[0075] The operating conditions of T1 and T2 towers are as follows:
[0076] The first reactive distillation column T1: column diameter 1000mm; column height 19000mm; the number of plates in the public rectification section, initial distillation section, side line section, and public stripping section are 10, 20, 30, 15 respectively; the internal pressure of the column is 0.5 MPa; tower top temperature 130°C; tower bottom temperature of common stripping section 190°C; tower bottom temperature of side line section 170°C.
[0077] Atmospheric distillation column T2: column diameter 4000mm; column he...
Embodiment 2
[0080] The operating conditions of T3 and T4 towers are as follows:
[0081] Vacuum distillation tower T3: tower diameter 3000mm; tower height 40000mm; the number of plates in the public rectification section, initial distillation section, side line section, and public stripping section are 10, 20, 30, 15 respectively; the pressure inside the tower is 0.1 MPa The temperature at the top of the tower is 90°C, and the temperature at the bottom of the common stripping section is 160°C; the temperature at the bottom of the side line section is 120°C.
[0082] Vacuum distillation tower T4: tower diameter 3000mm; tower height 40000mm; the number of plates in the public rectification section, initial distillation section, side line section and public stripping section are 10, 20, 30, 15 respectively; the pressure inside the tower is 0.1 MPa ; The temperature at the top of the tower is 165°C, and the temperature at the bottom of the common stripping section is 180°C; the temperature at...
Embodiment 3
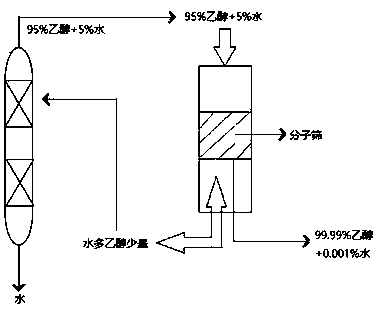
[0085] The 95% ethanol obtained from the top of the T2 atmospheric distillation tower is dewatered by a 13X molecular sieve dewatering device to obtain 99.99% ethanol and returned to the bottom of the T1 tower to continue the rectification reaction.
[0086] 95% ethanol is pumped into the molecular sieve water removal device at a certain speed to obtain 99.99% ethanol. Use a vacuum pump to pump a large amount of water and a small amount of ethanol above the molecular sieve into the vacuum distillation tower. A large amount of water can be obtained at the bottom of the tower, and 95% ethanol can be obtained at the top of the tower. % ethanol, which is pumped into the molecular sieve water removal device for recycling.
[0087] Diagram of molecular sieve water removal device figure 2 Shown:
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com