Method for preparing aluminum nitride ceramic powder based on urea/melamine nitrogen source
A technology of melamine nitrogen source and aluminum nitride ceramics, which is applied in the field of materials, can solve problems such as hindering nitrogen diffusion, particle growth, and aluminum frit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
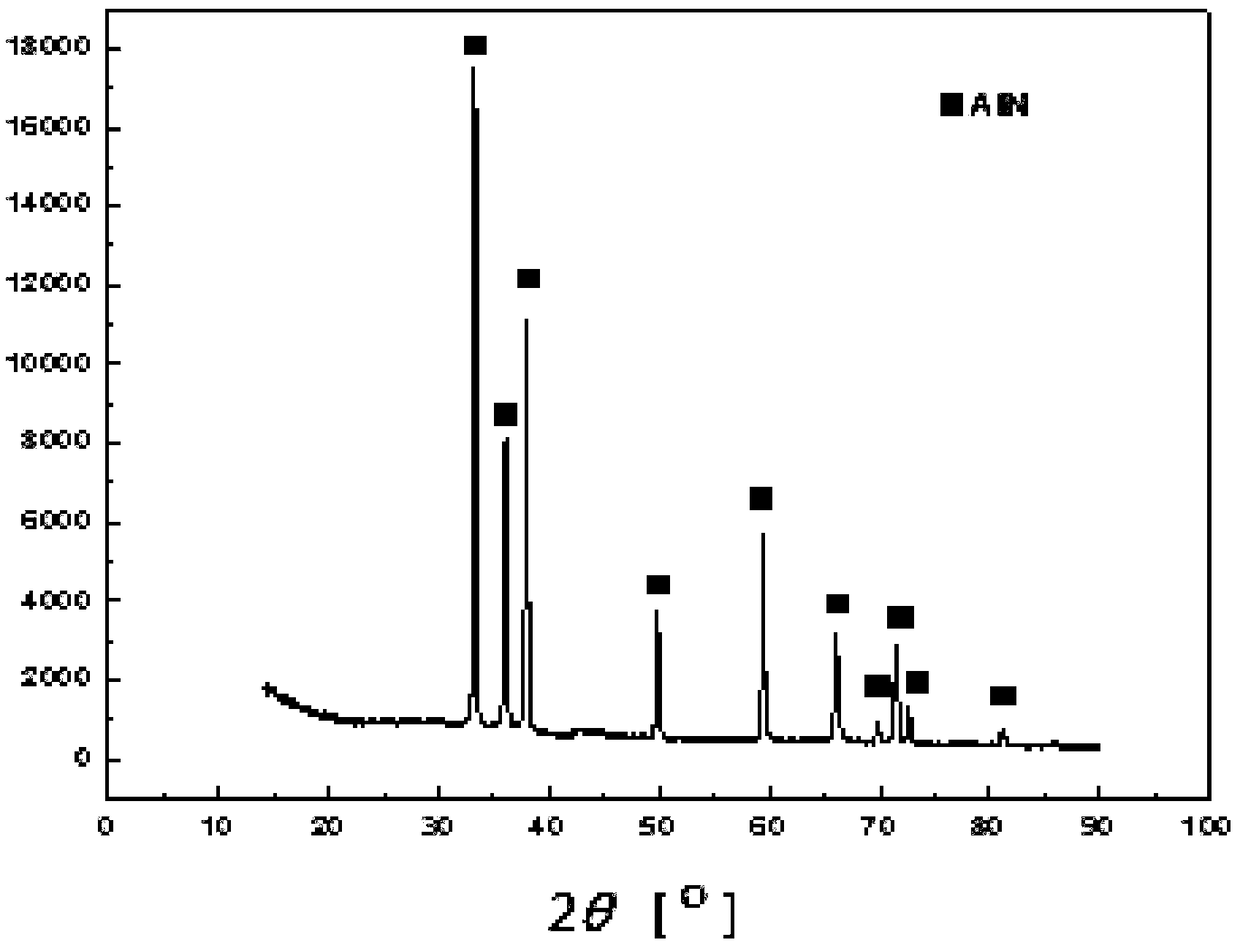
Image
Examples
Embodiment 1
[0034] Prepare aluminum source nonahydrate aluminum nitrate, precipitation agent ammonium bicarbonate solution, concentration of 0.1M; surfactant coupling agent and polyethylene glycol, carbon source phenolic resin, nitrogen source urea; wherein the coupling agent is nonahydrate aluminum nitrate 0.01% of the total mass, polyethylene glycol is 0.011% of the total mass of aluminum nitrate nonahydrate, the molar ratio of phenolic resin to aluminum nitrate nonahydrate is 1.2, the molar ratio of urea to aluminum nitrate nonahydrate is 3, the amount of precipitation agent The molar ratio of ammonium bicarbonate to aluminum nitrate nonahydrate is 8; the polymerization degree of polyethylene glycol is 6000;
[0035] Dissolve aluminum nitrate nonahydrate in deionized water, then add coupling agent and polyethylene glycol, and stir to make a mixed solution; the amount of deionized water can completely dissolve aluminum nitrate nonahydrate, coupling agent and polyethylene glycol Prevail
[0...
Embodiment 2
[0041] The method is the same as in Example 1, the difference is:
[0042] (1) The precipitation agent is ammonia water with a concentration of 0.1M; the nitrogen source is melamine; the coupling agent is 1% of the total mass of aluminum nitrate nonahydrate, polyethylene glycol is 1% of the total mass of aluminum nitrate nonahydrate, phenolic resin and The molar ratio of aluminum nitrate nonahydrate is 3, the molar ratio of melamine to aluminum nitrate nonahydrate is 6, and the amount of precipitant is NH 3 ·H 2 The molar ratio of O to aluminum nitrate nonahydrate is 8; the degree of polymerization of polyethylene glycol is 8000;
[0043] (2) Drying temperature 80℃, time 10 hours; calcination temperature 400℃, time 2 hours;
[0044] (3) Nitriding synthesis reaction temperature is 1000℃, time is 2 hours;
[0045] (4) The coarse aluminum nitride powder is kept at 620°C for 4 hours to remove carbon; the particle size of the aluminum nitride ceramic powder is 280-430 nanometers.
Embodiment 3
[0047] The method is the same as in Example 1, the difference is:
[0048] (1) The concentration of the precipitation agent ammonium bicarbonate solution is 2M; the coupling agent is 5% of the total mass of aluminum nitrate nonahydrate, polyethylene glycol is 5% of the total mass of aluminum nitrate nonahydrate, phenolic resin and aluminum nitrate nonahydrate The molar ratio of urea is 6, the molar ratio of urea to aluminum nitrate nonahydrate is 12, the amount of precipitant is 2 according to the molar ratio of ammonium bicarbonate to aluminum nitrate nonahydrate; the degree of polymerization of polyethylene glycol is 20,000;
[0049] (2) Drying temperature 60℃, time 48 hours; calcination temperature 300℃, time 4 hours;
[0050] (3) Nitriding synthesis reaction temperature is 1050℃, time is 2 hours;
[0051] (4) The coarse aluminum nitride powder is kept at 600°C for 3 hours to remove carbon; the particle size of the aluminum nitride ceramic powder is 300-500 nanometers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com