STa-LTB-STb fusion protein of enterotoxin of escherichia coli and encoding gene and application thereof
A fusion protein and fusion gene technology, applied in the biological field, can solve the problems of not being able to fight against pathogenic factors-enterotoxin, the narrow protection scope of vaccines, etc., and achieve the effects of improving the protection effect, preventing harm, and simplifying the protein purification process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Construction of STa-LTB-STb fusion gene
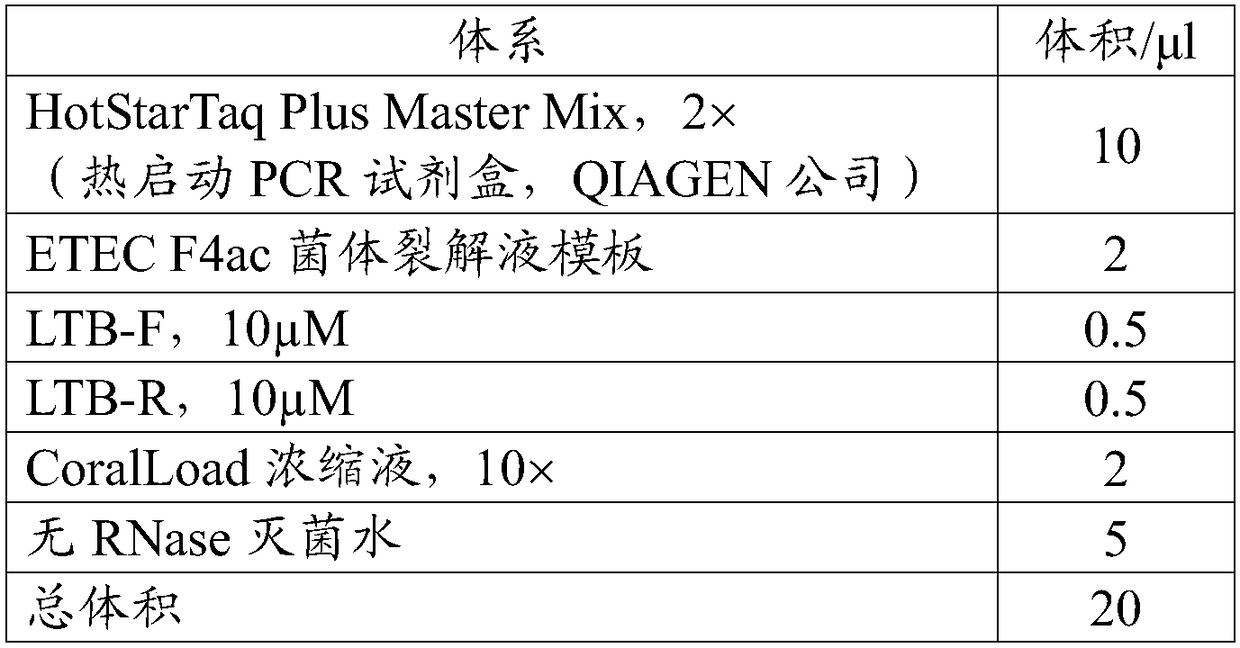
[0101] (1), enterotoxin gene LTB PCR amplification
[0102] The PCR reaction template is ETEC F4ac cell lysate (the strain was purchased from the China Veterinary Drug Control Institute, the number is C83902), and the primers, reaction system and reaction conditions used are as follows:
[0103] Primers:
[0104] name
[0105] reaction system:
[0106]
[0107] Reaction conditions: 95°C for 5 min, (94°C for 30 s, 55°C for 30 s, 72°C for 1 min) amplification for 30 cycles, and 72°C for 10 min.
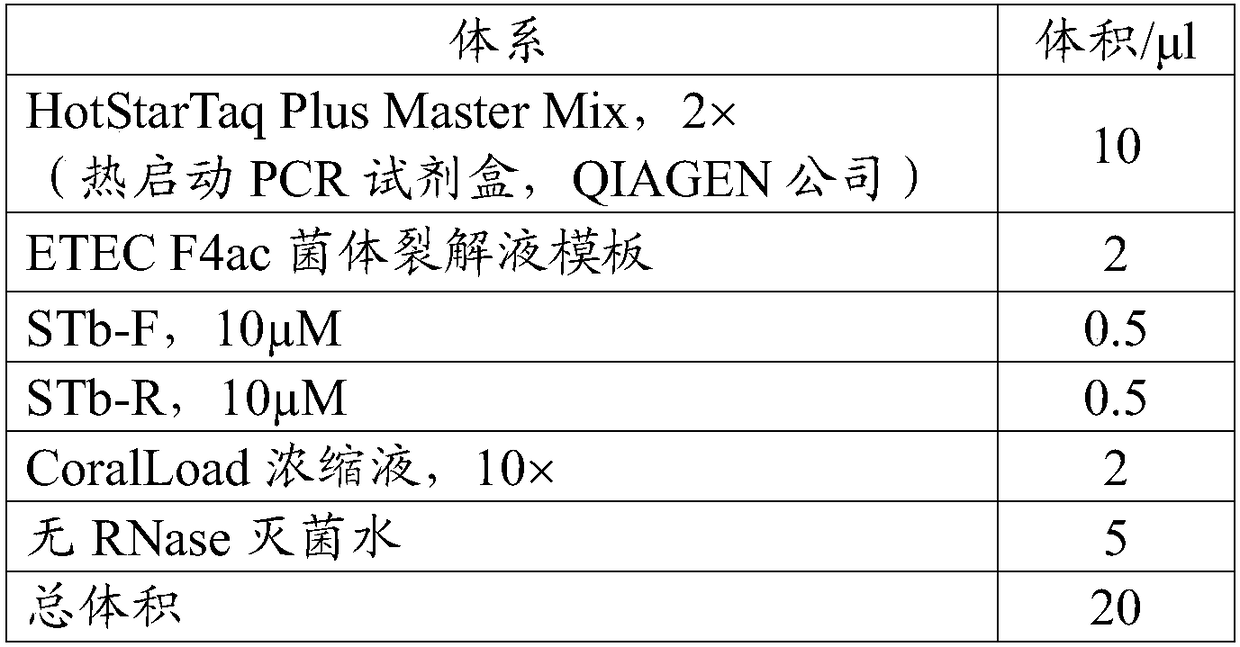
[0108] (2), enterotoxin gene STb PCR amplification
[0109] Primers:
[0110] name
[0111] reaction system:
[0112]
[0113] Reaction conditions: 95°C for 5 min, (94°C for 30 s, 55°C for 30 s, 72°C for 1 min) amplification for 30 cycles, and 72°C for 10 min.
[0114] (3), enterotoxin gene STa PCR amplification
[0115] The size of the STa gene is only 54bp. According to the principle of overlap extension...
Embodiment 2
[0136] Eukaryotic Expression of Enterotoxin STa-LTB-STb Fusion Protein
[0137] (1) Construction of eukaryotic expression vector of enterotoxin STa-LTB-STb fusion gene
[0138] Using the principles and methods of molecular biology, the STa-LTB-STb fusion gene was first connected to the pMD-19T cloning vector (full name: pMD TM -19T Vector Cloning Kit (manufacturer: Takara Company) to obtain the recombinant cloning T vector, and use the heat shock method to introduce the recombinant T vector into E.coli DH5α competent cells. The specific operation is: take 100 μl E.coli DH5α competent cells and add 5 μl Recombinant plasmid (recombinant clone T vector), ice bath for 30min, heat shock in 42°C water bath for 90s, place on ice for 2min, add 500μl LB liquid medium and mix well, take 200μl and spread on LB plate containing Amp (60μg / ml) above, cultured at 37°C for 12 hours, picked a single colony and cultured it in LB liquid medium to ensure the integrity of the gene fragment; extra...
Embodiment 3
[0161] Evaluation of the safety and immune protection effect of enterotoxin STa-LTB-STb fusion protein on experimental animals
[0162] (1) Ultrafiltration concentration of recombinant enterotoxin STa-LTB-STb fusion protein
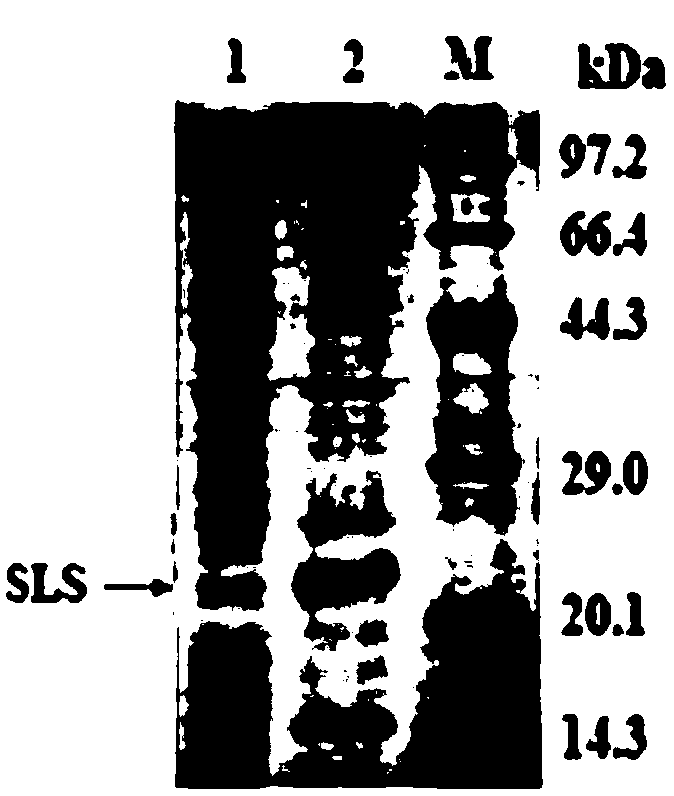
[0163] Concentrate the supernatant from the induced culture of the recombinant strain of Pichia pastoris using an ultrafiltration tube, and operate according to the instructions of the Amicon Ultra-15 ultrafiltration centrifuge tube produced by Millipore, USA. First, the culture medium is centrifuged at 2000rpm for 5min; the obtained supernatant is added to an ultrafiltration tube, centrifuged at 4000rpm for 25min, the filtrate is discarded, and the collected solution is stored for later use, which is enterotoxin STa-LTB-STb Fusion protein solution, the SDS-PAGE electrophoresis figure of described solution is as follows figure 1 shown.
[0164] (2) Preparation of recombinant enterotoxin STa-LTB-STb fusion protein vaccine
[0165] The recombinant entero...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com