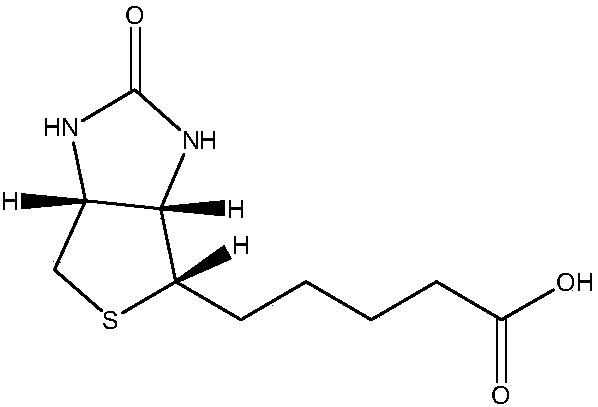
Preparation method of 5-(2-oxytetrahydrothiophene imidazole-4(2H)-alkenyl pentanoic acid compounds
A technology of benzimidazole and phenyltetrahydroimidazole is applied in the field of preparation of d-biotin intermediate 5-alkenyl)valeric acid compounds, which can solve serious side reactions, difficult separation and purification, and many reaction impurities. problems, to achieve the effect of easy separation, good safety and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
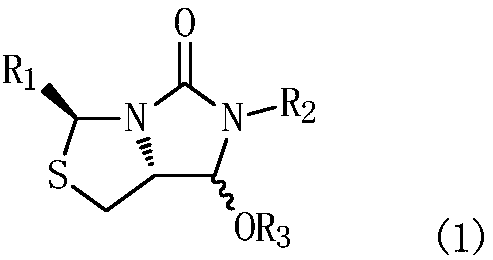
[0063] Example 1: (3S,7aR)-6-benzyl-7-(2-oxocyclohexyl)-3-phenyltetrahydroimidazo[1,5-c]thiazol-5(3H)-one preparation
[0064] In a 500mL three-necked flask equipped with mechanical stirring and a thermometer, add 200mL of dichloromethane, and drop 32.6g (0.1mol) of compound (1a) (R 1 = phenyl, R 2 = benzyl, R 3 =H), add 18.7g (0.11mol) of 1-cyclohexenyloxytrimethylsilane, protect with nitrogen, stir and cool to -45°C, add 14.2g (0.1mol) of boron trifluoride diethyl ether dropwise in half an hour, Control the temperature at -45~-10℃, stir and react for 2~3 hours; after the reaction is completed, raise the temperature to -5~0℃, add water 50mL dropwise, separate layers, add dichloromethane 100mL×2 to extract twice, combine dichloromethane The methane organic phase was washed twice with saturated aqueous sodium bicarbonate solution 50mL×2, the above dichloromethane layer was evaporated, and the product was dried to obtain the target product (2a)(3S,7aR)-6-benzyl-7-(2 -Oxocycl...
Embodiment 14
[0066] Example 14: 6-((3S,7aR)-6-benzyl-5-oxo-3-phenylhexahydroimidazo[1,5-c]thiazol-7-yl-6-oxohexanoic acid Sodium preparation
[0067] In a 500mL autoclave with mechanical stirring and a thermometer, add 300mL of ethanol, and put the compound (2a)(3S,7aR)-6-benzyl-7-(2-oxocyclohexyl)- 3-Phenyltetrahydroimidazo[1,5-c]thiazol-5(3H)-one 40.6g(0.1mol)(R 1 = phenyl, R 2 = benzyl), add 4.0g (0.1mol) of sodium hydroxide, 26.2g (0.1mol) of cocatalyst triphenylphosphine, feed air to 0.3MPa (gauge pressure), stir and heat up to 40°C for reaction, with the reaction If the pressure drops, continue to add air. When the pressure does not drop, release the pressure to 0MPa (gauge pressure), and refill the air to 0.3MPa (gauge pressure). If the pressure does not drop, the previous reaction is completed (oxygen It is not necessary to release the pressure until the early reaction is completed). Remove the pressure, add 0.4g (0.005mol) of manganese dioxide, feed air until the reaction is c...
Embodiment 27
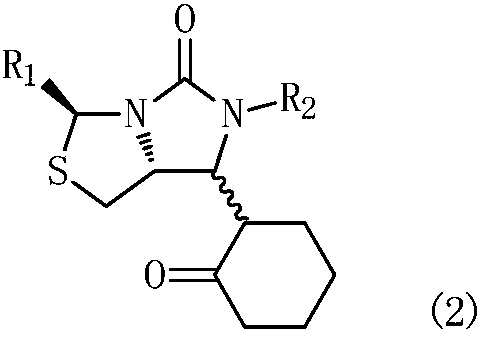
[0069] Example 27: Preparation of 6-((5R)-1,3-dibenzyl-5-(mercaptomethyl)-2-oxoimidazol-4-yl)-6-oxohexanoic acid
[0070] In a 500mL three-neck flask with mechanical stirring and a thermometer, put the compound (3a) 6-((3S,7aR)-6-benzyl-5-oxo-3-phenylhexahydro Imidazo[1,5-c]thiazol-7-yl-6-oxohexanoic acid sodium aqueous solution 320mL, containing compound (3a) 46.0g (0.1mol) (R 1 = phenyl, R 2 = benzyl), add 32.7g (0.5mol) of 500 mesh zinc powder at room temperature, protect with nitrogen, stir and heat up to 40°C, add dropwise 30% NaOH (26.6g, 0.2mol), control the pH value at 11.0~13.0, control The temperature is 40-45°C, stirring and reacting for 5-6 hours; after the reaction is completed, remove it by hot filtration, wash the filter cake with 100mL water, adjust the pH value of the filtrate to 3.5-4.0 with 30% HCl solution, add 200mL×2 toluene to extract twice , the toluene extract was washed twice with 50mL×2 water to obtain the target product (4a) 6-((5R)-1,3-dibenzyl-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com