Cobalt phosphide nano frame and preparation and application thereof
A cobalt phosphide, nano technology, applied in the field of electrolysis of water, to achieve the effect of high atomic utilization, good stability, high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Synthesis of CoP NF and CoP NC
[0065] (1) Synthesis of Co-Co PBA NC
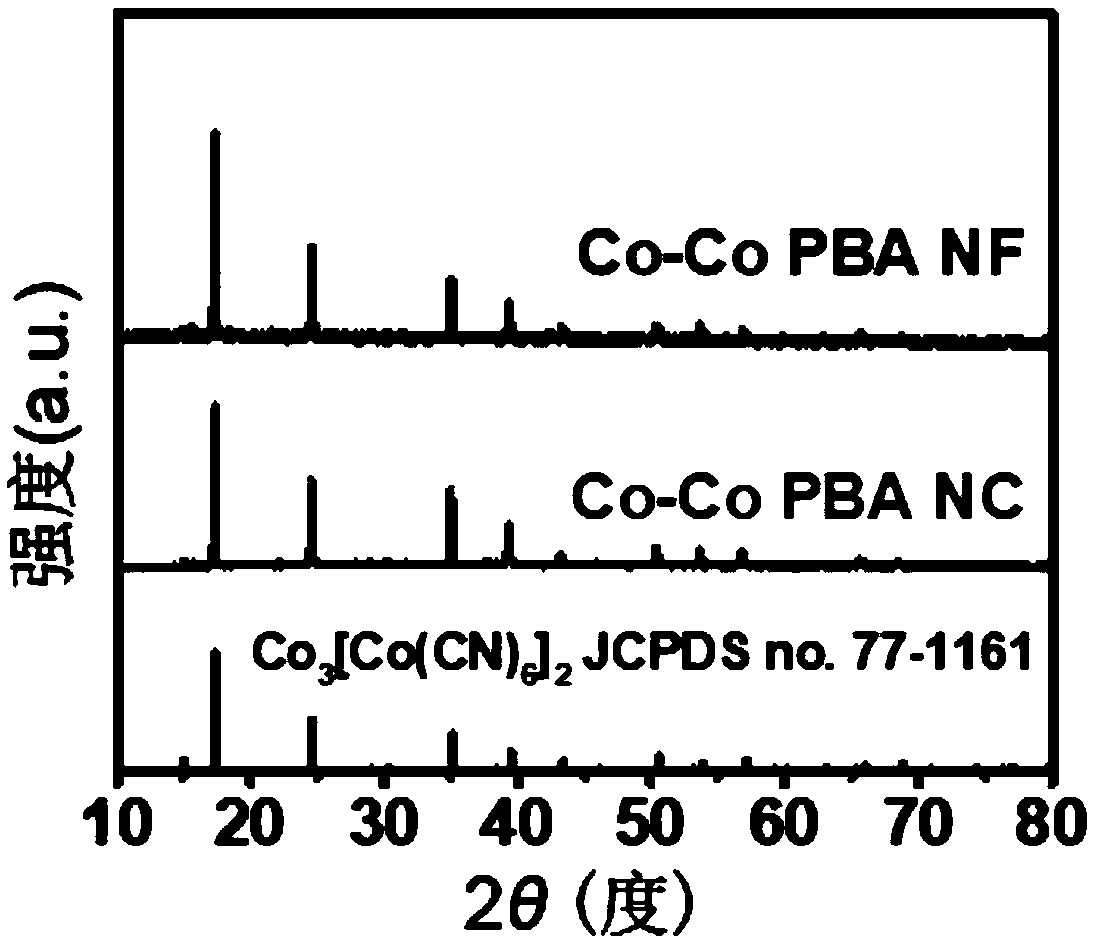
[0066] co 3 [Co(CN) 6 ] 2 Nanocubes (Co-Co PBA NCs) were prepared by a simple precipitation method.
[0067] 0.6mmol of cobalt acetate and 0.9mmol of sodium citrate were first dissolved in 20ml of deionized water to form solution A, while 0.4mmol of potassium cobaltcyanide was dissolved in 20ml of deionized water to form solution B. Subsequently, solution A and solution B were mixed and stirred for 3 min, and the mixed solution was allowed to stand at room temperature for 8 h. After the reaction, the product was centrifuged, washed, and vacuum-dried to obtain pink powder Co-Co PBA NC.
[0068] (2) Synthesis of Co-Co PBA NF
[0069] Co-Co PBA NFs were fabricated by chemically etching Co-Co PBA NCs.
[0070] Dissolve 5ml of ammonia water (28–30%) in 15ml of deionized water to form solution C, and ultrasonically disperse 20mg of Co-Co PBA NC in 10ml of ethanol to form solution D. Subsequently, ...
Embodiment 2
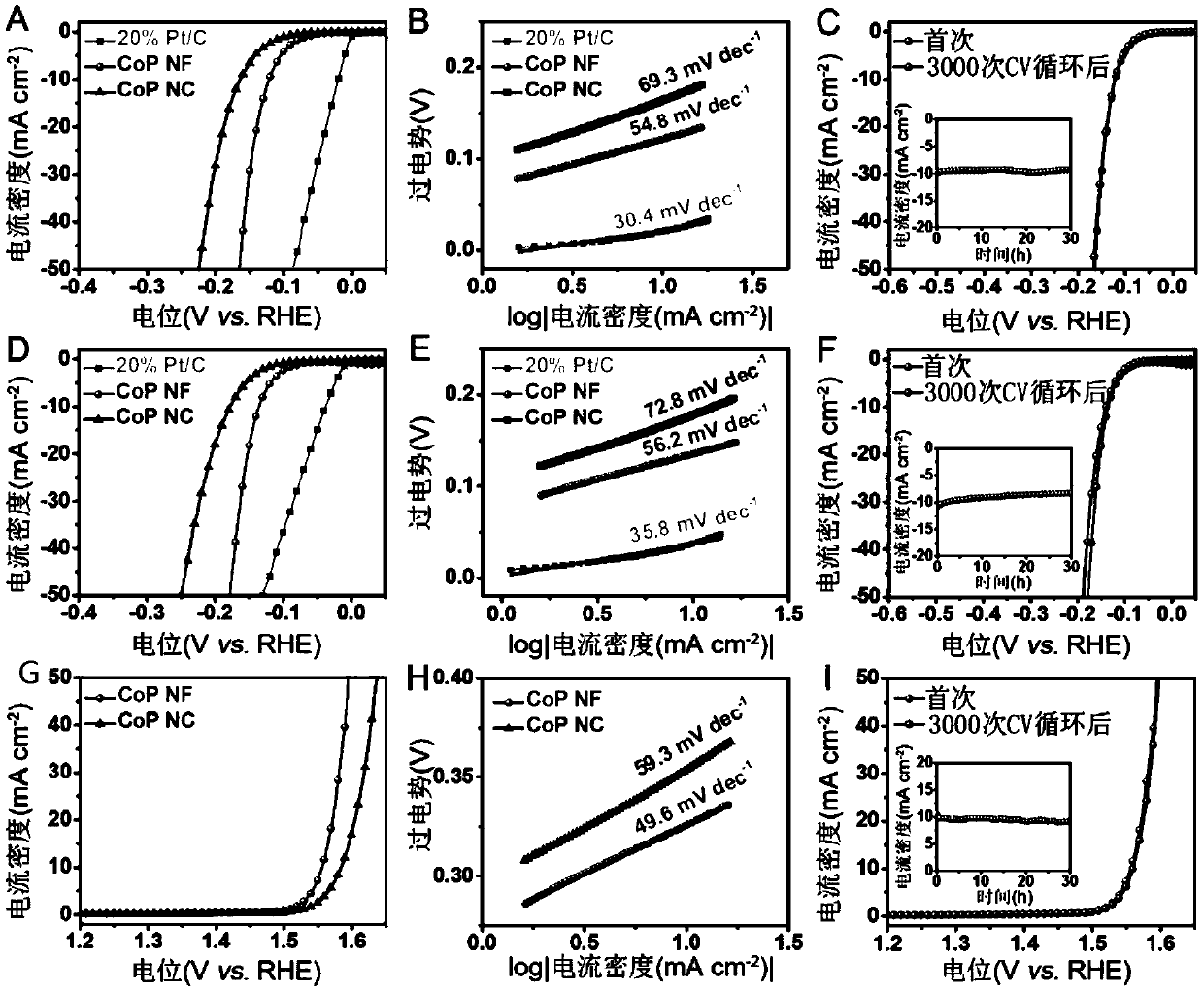
[0080] The CoP NF and CoP NC prepared in Example 1 were used as HER and OER electrocatalysts to prepare working electrodes respectively, and the specific steps were as follows:
[0081] 4 mg of electrocatalyst and 80 μl of 5wt% Nafion solution were first dispersed in 1ml of deionized water / ethanol mixed solution (v / v=4 / 1) to form a uniform electrocatalyst suspension, and 5 μl of the suspension was dropped on a glassy carbon electrode (diameter 3mm ) surface, after drying at room temperature, the electrocatalyst loading was about 0.265 mg cm –2 the working electrode. The same procedure was used to prepare a commercial 20% Pt / C electrode with the same loading.
[0082] The electrochemical performance test of the electrocatalyst was carried out in a standard three-electrode cell, the glassy carbon electrode loaded with the electrocatalyst was used as the working electrode, carbon rod (for HER) or Pt wire (for OER) was used as the counter electrode, and SCE was used as the refere...
Embodiment 3
[0088] Example 3: The CoP NF and CoP NC prepared in Example 1 were used to construct a two-electrode system, and then the electrocatalytic performance of the full hydrolysis was tested. The specific steps are as follows:
[0089] CoP NF was first drop-coated on carbon cloth (with a loading of 2 mg cm –2 ), and simultaneously serve as anode and cathode to construct a CoPNF||CoP NF two-electrode system. As a comparison, the CoP NC||CoP NC two-electrode system was also prepared using CoP NC as the electrocatalyst.
[0090] Such as Figure 28 As shown, in 1M KOH solution, CoP NF||CoP NF and CoP NC||CoP NC require voltages of 1.65 and 1.75V, respectively, to generate 10mA cm –2 The full hydrolysis catalytic current density and lower voltage again prove the higher electrocatalytic activity of CoPNF. When used as a bifunctional electrocatalyst for electrocatalytic perhydrolysis, the CoP NF exhibits excellent electrocatalytic performance, surpassing most of the reported non-preciou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com