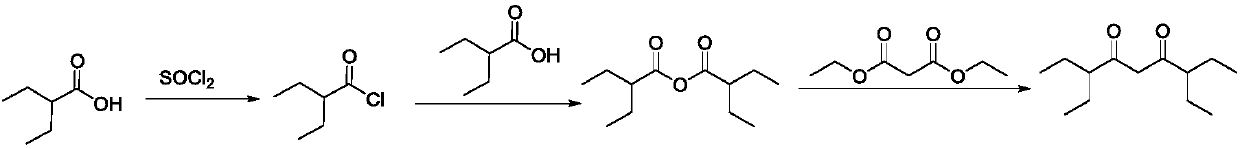
Preparation method of 3,7-diethylnonane-4,6-dione
A technology of ethyl nonane and diketone, which is applied in the field of compound preparation, can solve the problems of long-term high-temperature reaction and low-temperature reaction, cumbersome operation steps, and short synthesis process, and achieve mild reaction conditions, convenient operation steps, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
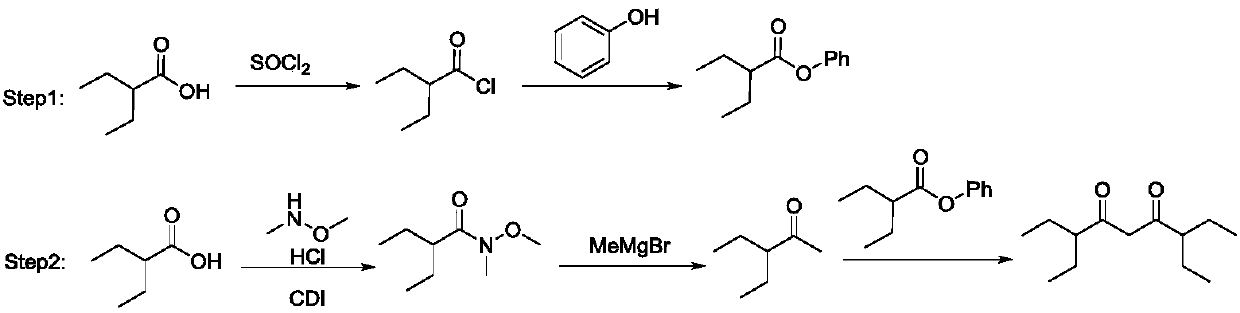
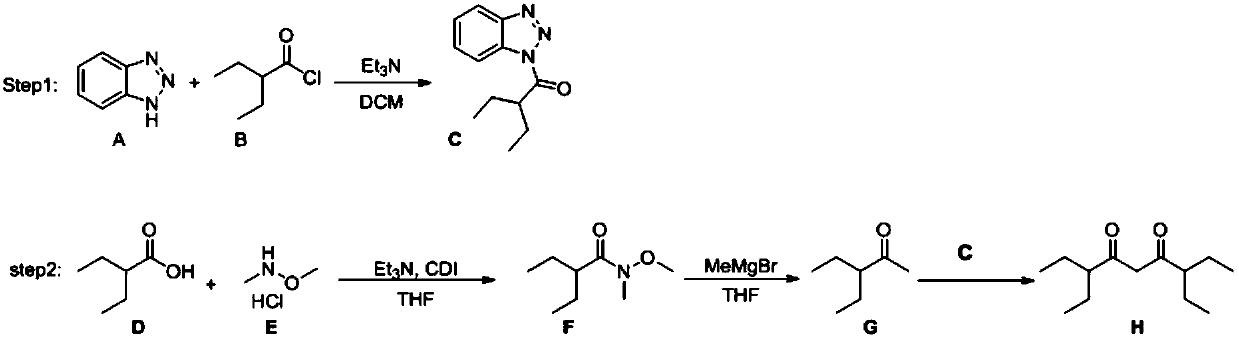
[0029] a: Dissolve 1.64g of raw material benzotriazole (14mmol) in 20mL of dichloromethane at 0°C, and add 2.3mL of triethylamine (16mmol) into the reaction system, then drop into 1.93mL of raw material 2 -Ethylbutyryl chloride (14mmol), stirred at 0°C for 4h, after quenching the reaction with 10% HCl solution, continued to stir for 15min, extracted with dichloromethane, and separated by column chromatography to obtain 2.6g of the product 1- Benzotriazole-2-ethyl-1-butanone, the yield is 86%;
[0030] b: Add 5.81g of raw material 2-ethylbutyric acid (50mmol) and 9.75g of N,N'-carbonyldiimidazole (60mmol) into a 100mL reaction flask, dissolve in 20mL of tetrahydrofuran, stir at room temperature for 0.5h, 2-ethyl The reactant of butyric acid and N,N'-carbonyldiimidazole can be reacted more easily, and 5.85g of dimethylhydroxylamine hydrochloride (60mmol) and 7.08g of triethylamine (70mmol) were added in another 50mL reaction bottle , dissolved in 20mL tetrahydrofuran, stirred a...
Embodiment 2
[0034] In a 25mL reaction flask, the 1.15g product 1-benzotriazole-2-ethyl-1-butanone (5.3mmol) and catalyst obtained in step a of Example 1 were dissolved in dichloromethane, Then 0.41 g of the product 3-ethyl-2-pentanone (3.6 mmol) obtained in step c of Example 1 was added dropwise to the reaction system, stirred at room temperature for 3 h, and after quenching the reaction with 10% HCl solution, Extracted with dichloromethane, and separated by column chromatography to obtain 0.47 g of the product 3,7-diethylnonane-4,6-dione, with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com