A kind of preparation method of anidungin side chain intermediate p-pentyloxyterphenylcarboxylic acid
A technology of p-pentoxy terphenyl formic acid and n-pentyloxy biphenyl is applied in the field of preparation of p-pentyloxy terphenyl acid in the side chain of anidungin, and can solve the problems of complicated operation, difficult control and high risk. , to achieve the effect of simple and safe process operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
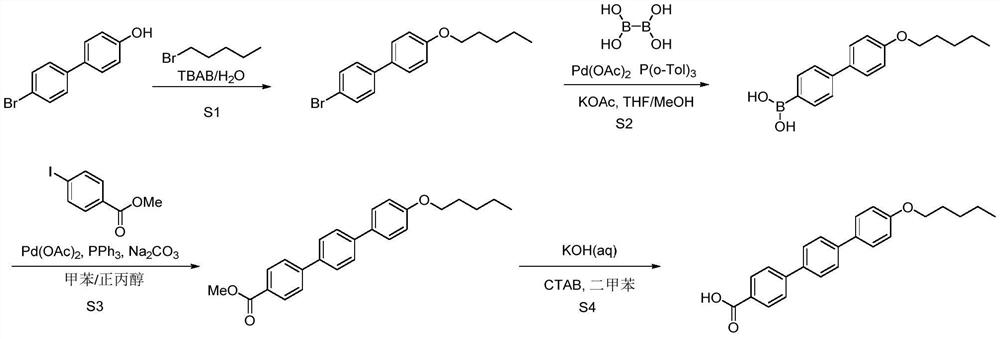
[0076] Example 1 Synthesis of 4'-bromo-4-n-pentoxybiphenyl
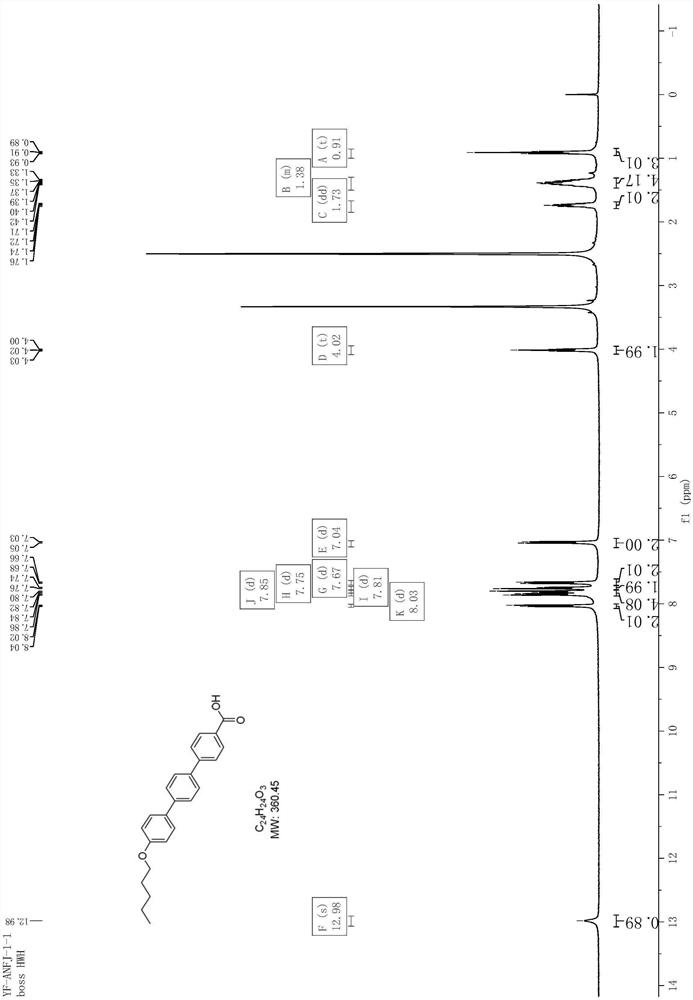
[0077] Add 1450mL of water and 21.2g of sodium hydroxide to a 2L three-necked flask, stir to dissolve, add 120g of 4-hydroxy-4'-bromobiphenyl and 6g of tetrabutylammonium bromide, stir at room temperature for 10min, then add 87.2g dropwise 1-Bromopentane, reflux for 5 hours after the dropwise addition, and filter with suction after the reaction has dropped to room temperature. After filtering and washing the filter cake with 60 mL of n-heptane, the obtained solid was vacuum-dried at 60° C. for 5 hours, and the yield was 87%. 1 H NMR (d 6 DMSO,400MHz)δ8.04-8.02(m,2H),7.85-7.83(m,1H),7.63-7.57(m,3H),7.02-7.00(m,2H),4.01-3.98(m,2H) ,1.75(t,J=8.0Hz,2H),1.41-1.36(m,4H),0.92-0.89(m,3H).
Embodiment 2
[0078] Example 2 Synthesis of 4'-bromo-4-n-pentoxybiphenyl Add 1200mL of water and 17.7g of sodium hydroxide to a 2L three-necked flask, stir to dissolve and add 100g of 4-hydroxy-4'-bromobiphenyl and 5g of tetrabutylammonium bromide, stirred at room temperature for 10min, then added dropwise 72.7g of 1-bromopentane, refluxed for 5h after the dropwise addition, and suction filtered after the reaction was lowered to room temperature, and the filter cake was washed with 300mL of water, and the solid Heat slurry with 320mL n-heptane / water (1:1) mixed solution, filter with suction and wash the filter cake with 60mL n-heptane, and dry the obtained solid under vacuum at 60°C for 5 hours, with a yield of 87%. 1 H NMR (d 6 DMSO,400MHz)δ8.04-8.02(m,2H),7.85-7.83(m,1H),7.63-7.57(m,3H),7.02-7.00(m,2H),4.01-3.98(m,2H) ,1.75(t,J=8.0Hz,2H),1.41-1.36(m,4H),0.92-0.89(m,3H).
Embodiment 3
[0079] Example 3 Synthesis of 4-pentyloxy-4'-biphenylboronic acid Under nitrogen protection, add 5g of 4'-bromo-4-n-pentyloxybiphenyl, 88mg of palladium acetate, 4.6g of potassium acetate into a 100mL three-necked flask, 144mg of tris(o-methylphenyl)phosphine and 15mL of tetrahydrofuran, cooled to 0-10°C in an ice-water bath, 1.84g of tetrahydroxydiboron dissolved in 10mL of methanol and added dropwise to the reaction, stirred at room temperature, and added after the raw materials disappeared Dilute with 30 mL of ethanol, filter with suction, wash the filter cake with 10 mL of ethanol, spin the filtrate, add 80 mL of dichloromethane / water mixed solvent (1:1) for beating at 40°C for 1 hour, cool to room temperature and filter with suction, and filter the cake in turn Wash with 5 mL of water and 5 mL of dichloromethane, and dry in vacuo at 50° C. to obtain 2.84 g of a white solid with a yield of 64%. 1 H NMR (d 6 DMSO,400MHz)δ7.98-7.96(m,2H),7.85-7.83(m,2H),7.62-7.57(m,4H),7.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com