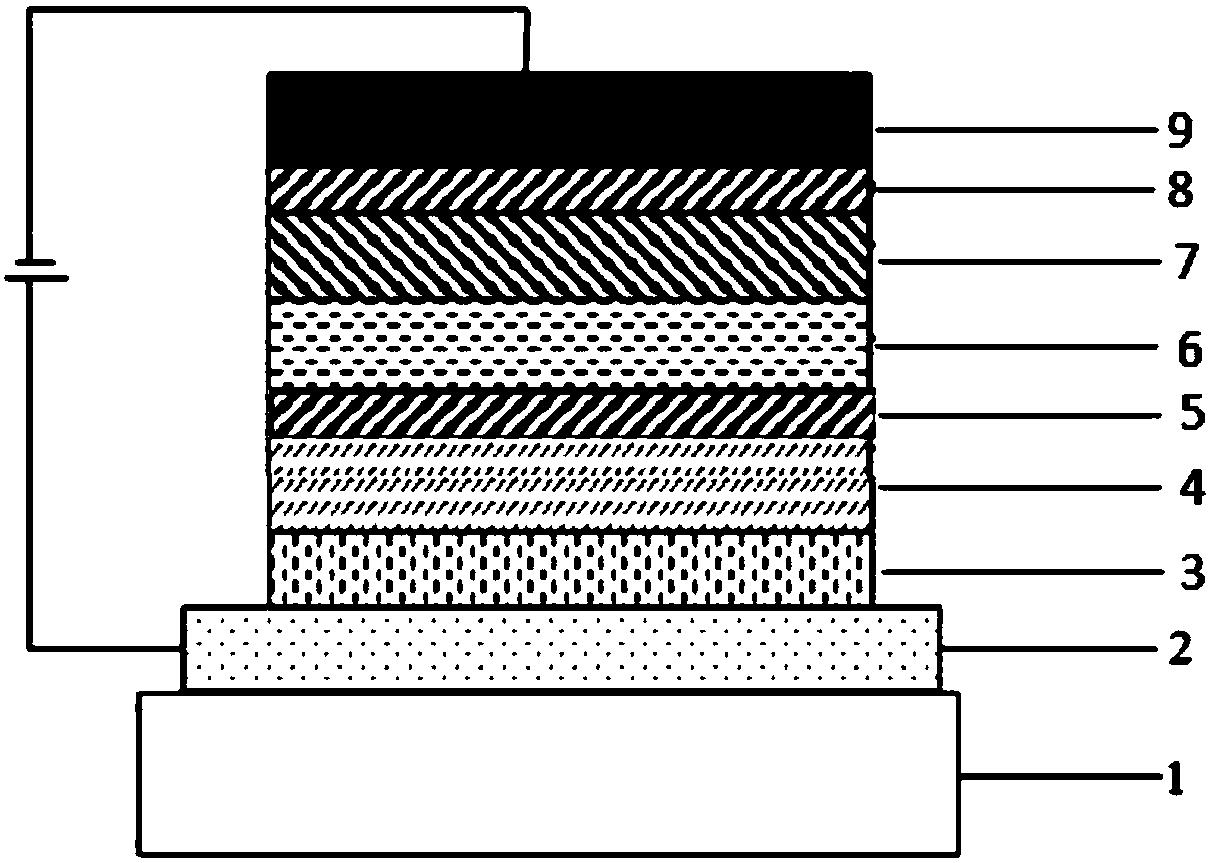
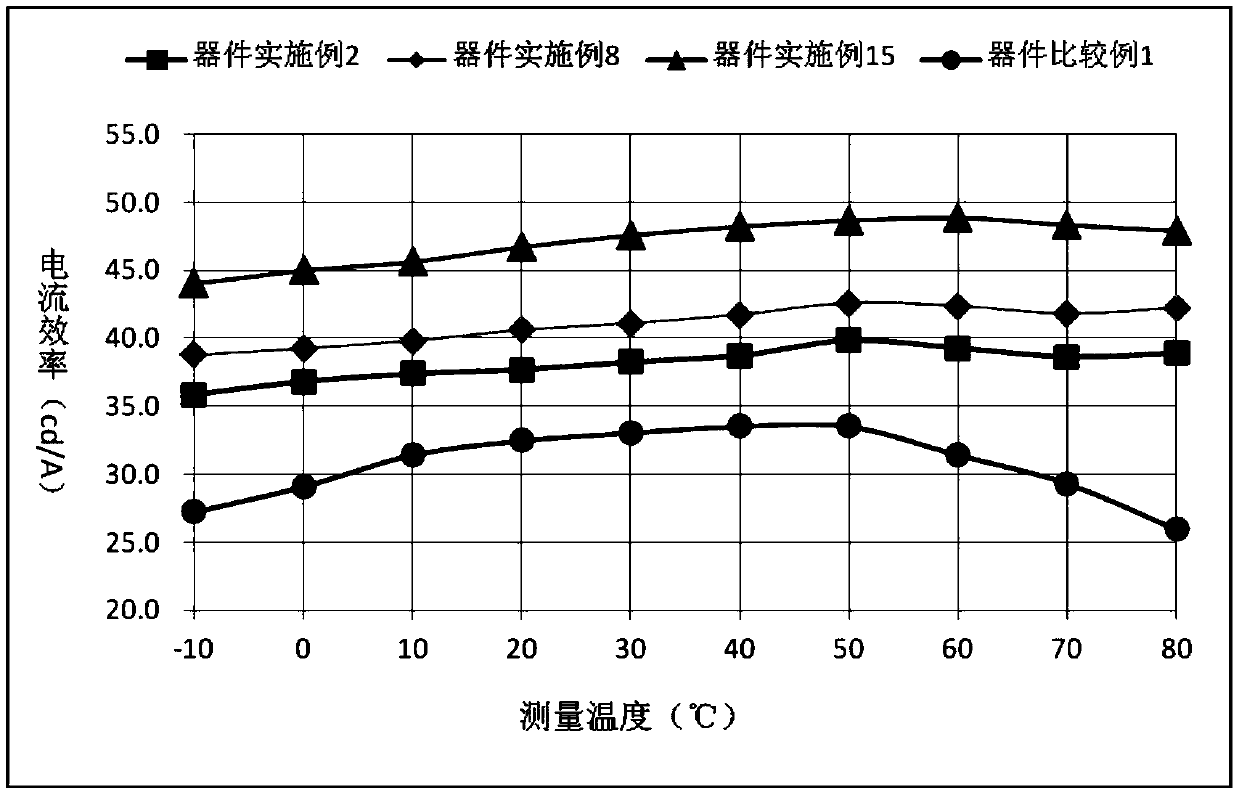
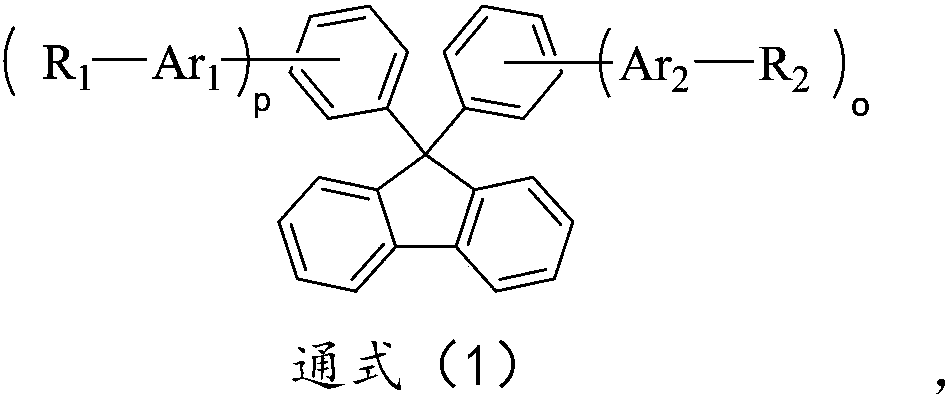
Nitrogen-containing seven-membered heterocyclic derivative compound, preparation method thereof and application of compound to organic light-emitting device
A compound and seven-membered ring technology, applied in the field of compounds containing nitrogen-containing seven-membered ring derivatives, can solve different problems, improve current efficiency and lifespan, improve high fluorescence radiation efficiency, and improve hole injection and transport performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: the synthesis of intermediate Cn:
[0081]
[0082] (1) In a 250mL three-necked flask, add 0.1mol raw material I, 0.15mol raw material J, 0.3mol sodium tert-butoxide, 1×10 -3 mol Pd 2 (dba) 3 , 1×10 -3 mol tri-tert-butylphosphine, 150mL toluene, heated to 95°C, refluxed for 24 hours, sampled and plated, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the intermediate Sn.
[0083] (2) In a 250mL three-neck flask, add 150mL of liquid ammonia, 0.05mol of intermediate Sn, and 0.15mol of potassium tert-butoxide under an atmosphere of nitrogen gas, place the reaction solution under a 500w xenon lamp for photochemical reaction for 1 hour, and then add nitric acid The reaction was quenched with ammonium, the residue was added to water after the liquid ammonia evaporated, the aqueous phase was extracted with dichloromethane, dried over anhydrous magnesium sulfate, the ...
Embodiment 2
[0084] Embodiment 2: the synthesis of intermediate Dn:
[0085]
[0086] (1) Weigh the raw material K and phenylboronic acid, dissolve them in a mixed solvent of toluene and ethanol with a volume ratio of (2~3):1, and add potassium carbonate aqueous solution and tetrakistriphenylphosphorous palladium under an inert atmosphere, at 95~110 Reaction at ℃ for 10-24 hours, cooled to room temperature, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the intermediate Tn; wherein the molar ratio of the raw material K to phenylboronic acid was 1: (1.2-1.5); the bromide raw material K and carbonic acid The molar ratio of potassium is 1:(2.0~3.0); the molar ratio of raw material K and tetrakistriphenylphosphorous palladium is 1:(0.01~0.02);
[0087] (2) Dissolve stannous chloride in 50mL of concentrated hydrochloric acid, slowly drop the concentrated hydrochloric acid solution of stannous chloride into the intermediate Tn, stir at room temp...
Embodiment 3
[0090] Embodiment 3: the synthesis of intermediate En:
[0091]
[0092]
[0093] (1) Weigh raw material M1 and raw material N1, add potassium phosphate aqueous solution, copper nanoparticles and toluene, react at 110°C for 2 hours, cool to room temperature, extract with ethyl acetate, wash with water, dry, pass through a silica gel column, Obtain intermediate Y1; wherein the molar ratio of raw material M1 to raw material N1 is 1:(1.0~1.4); the molar ratio of raw material M1 to potassium phosphate is 1:(2.0~3.0);
[0094] (2) In a 250mL three-necked flask, add methylmagnesium iodide and intermediate Y1 into tetrahydrofuran at 0°C under a nitrogen atmosphere, stir at room temperature for 2 hours, cool the reaction mixture to 0°C, add saturated ammonium chloride to quench The reaction was extinguished, stirred for 30 min, and evaporated in vacuum. The residue was extracted with chloroform, dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com