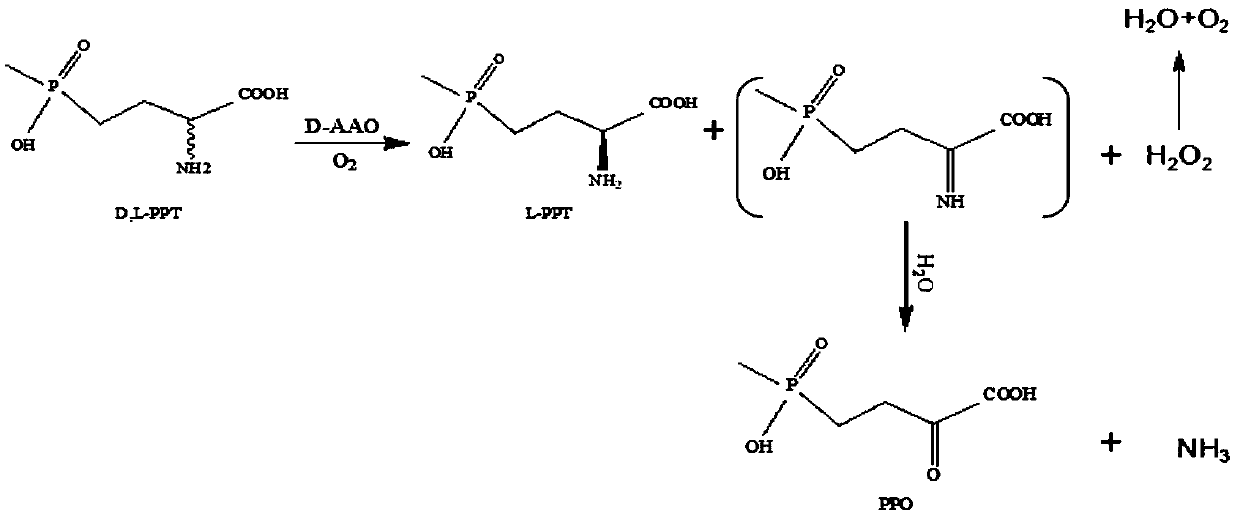
D-amino acid oxidase mutant and application thereof
An amino acid and oxidase technology, applied in the direction of oxidoreductase, application, enzyme, etc., can solve the problems of waste of raw materials, low single resolution rate, complicated process, etc., and achieve high yield, good application value, and tolerance high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Construction and screening of D-amino acid oxidase mutant library
[0057] The D-amino acid oxidase gene (the amino acid sequence shown in SEQ ID No.1, the nucleotide sequence shown in SEQ ID No.2) was constructed as an expression vector pET-24a(+), transformed into Escherichia coli, and the starting strain E.coli was obtained BL21(DE3) / pET-24a.
[0058] The D-amino acid oxidase mutant library was prepared through five rounds of mutation, and the primers were designed as shown in Table 1.
[0059] The specific method is as follows:
[0060] In the first round, using the genome of the wild fungus Rhodotorula gracilis as a template, using epPCR-F and epPCR-R as upstream and downstream primers, through error-prone PCR, transformation, smearing, screening of dominant strains, and sequencing to find Beneficial mutation sites 213, 54, 58, 52, 335 were identified.
[0061] In the second round, using the genome of the wild fungus Rhodotorula gracilis as a template,...
Embodiment 2
[0082] Embodiment 2 Construction of high-throughput screening method
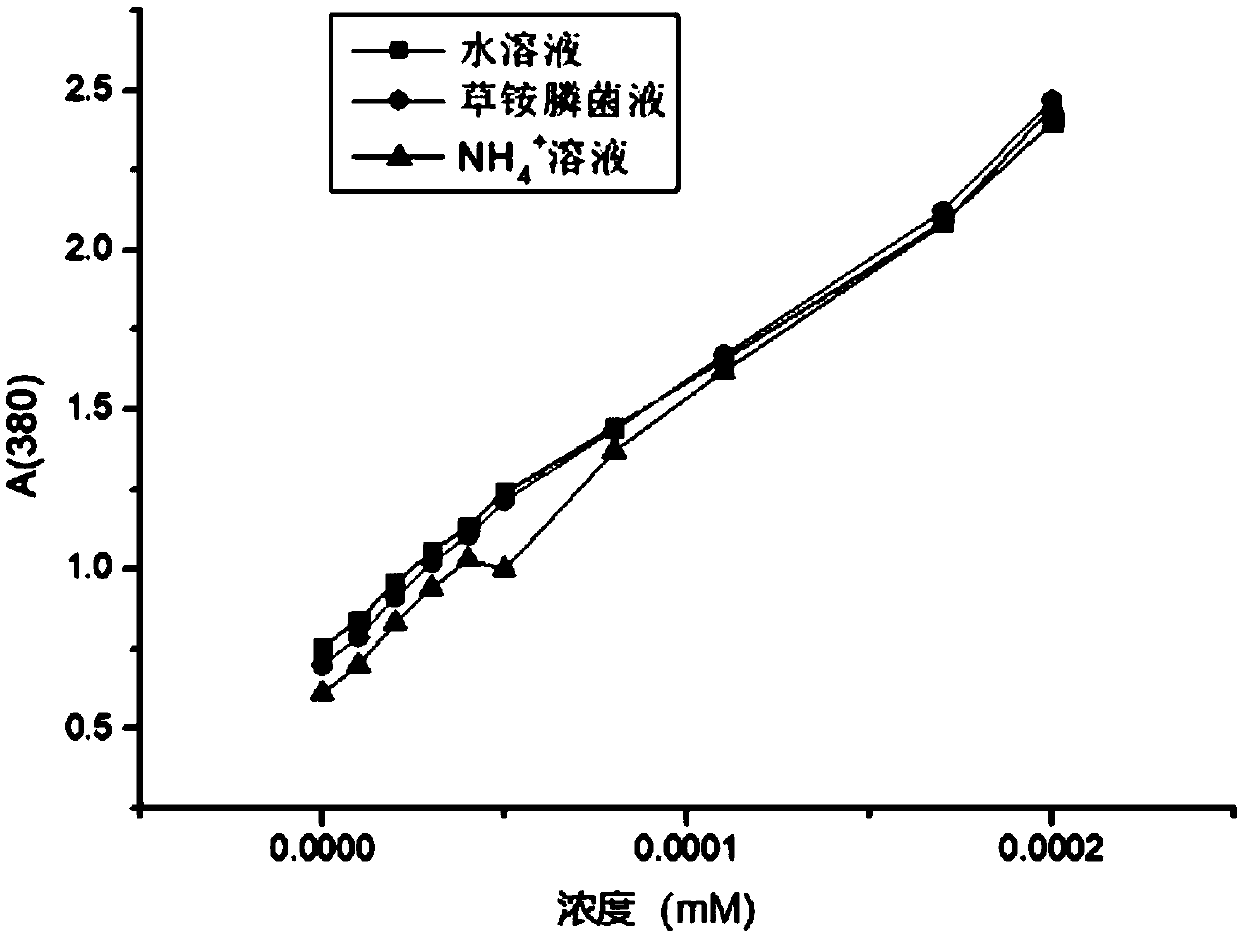
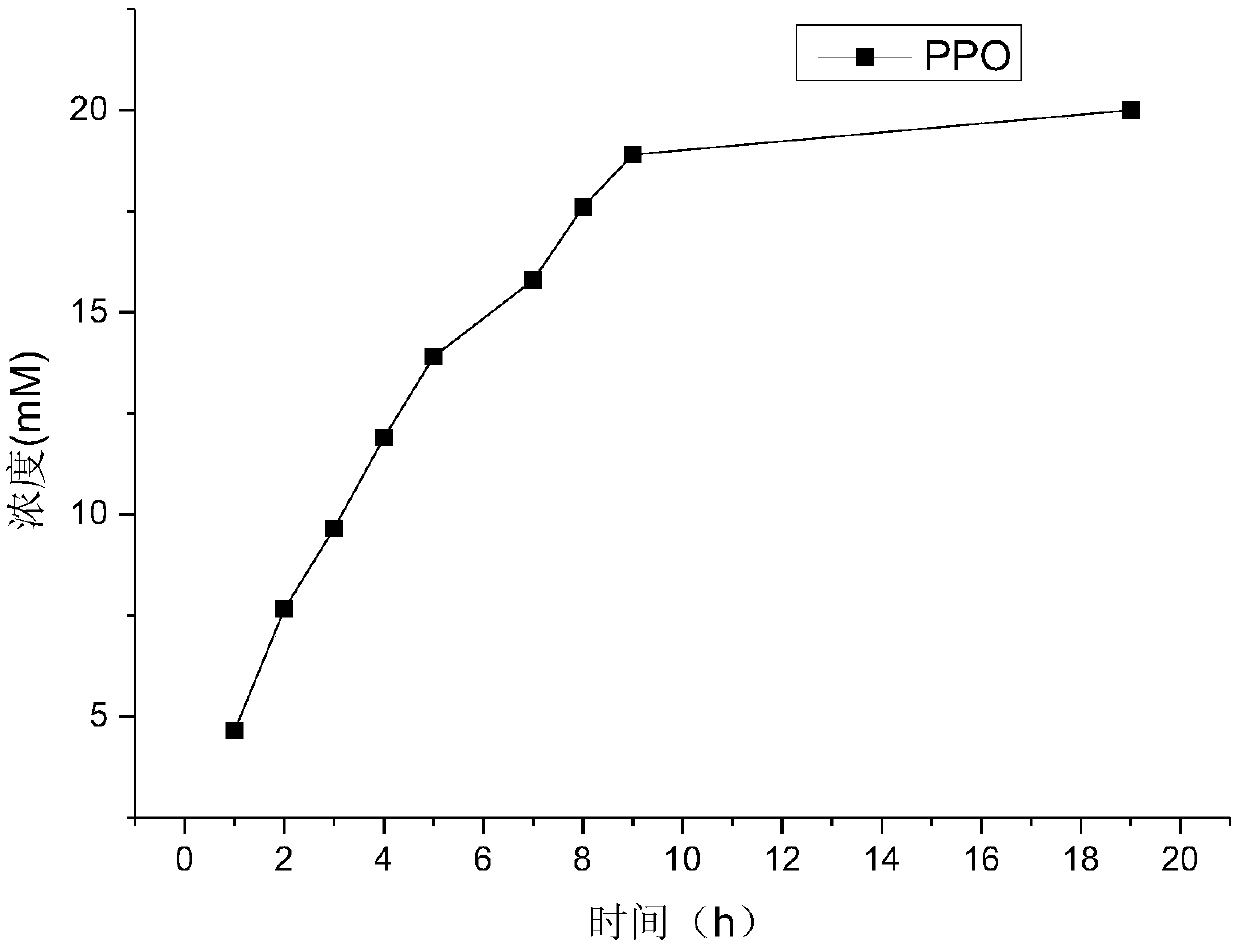
[0083] Using D,L-glufosinate-ammonium as substrate, enzymes with catalytic activity to D-glufosinate-ammonium were screened by 2,4-dinitrophenylhydrazine colorimetric method.
[0084] Quantitatively weigh D, L-glufosinate-ammonium into a 50mM phosphate buffer solution with pH=8, and place it in the reaction vessel so that the final concentration of D,L-glufosinate-ammonium is 50mM, and the concentration of the crude enzyme solution is 50g / L. The concentration of catalase was 2g / L. The reaction temperature was controlled at 30° C. by a water bath, and samples were taken regularly.
[0085] Detect the content of 2-carbonyl-4-[hydroxy(methyl)phosphono]butyric acid by 2,4-dinitrophenylhydrazine chromogenic method: take 60 μL of the reaction solution, add 2 mM 2,4-dinitro 40 μl of phenylhydrazine, incubated in a constant temperature bath at 37°C for 20 minutes, added 100 μl of 1M sodium hydroxide, and mixed fo...
Embodiment 3
[0093] The cultivation of embodiment 3 thalline and the purification of mutant
[0094] 1. Bacteria culture
[0095] After the engineering bacteria containing the D-amino acid oxidase gene were activated by streaking on a plate, a single colony was picked and inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 50mL LB liquid medium also containing 50μg / mL kanamycin according to 2% inoculum amount, and culture with shaking at 37°C until OD 600 When it reaches about 0.8, add IPTG with a final concentration of 0.5mM, and culture with shaking at 28°C for 16h. After the cultivation, the culture solution was centrifuged at 8000rpm for 10min, the supernatant was discarded, the bacteria were collected, and stored in a -80°C ultra-low temperature refrigerator until use.
[0096] 2. Preparation of crude enzyme solution
[0097] After the culture, the collected cells were washed twice with pH 8 phosphate buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com