Preparation method and application of silver/zinc oxide composite material
A composite material, zinc oxide technology, applied in chemical instruments and methods, catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem that the particle size of Ag nanoparticles is difficult to be effectively controlled.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
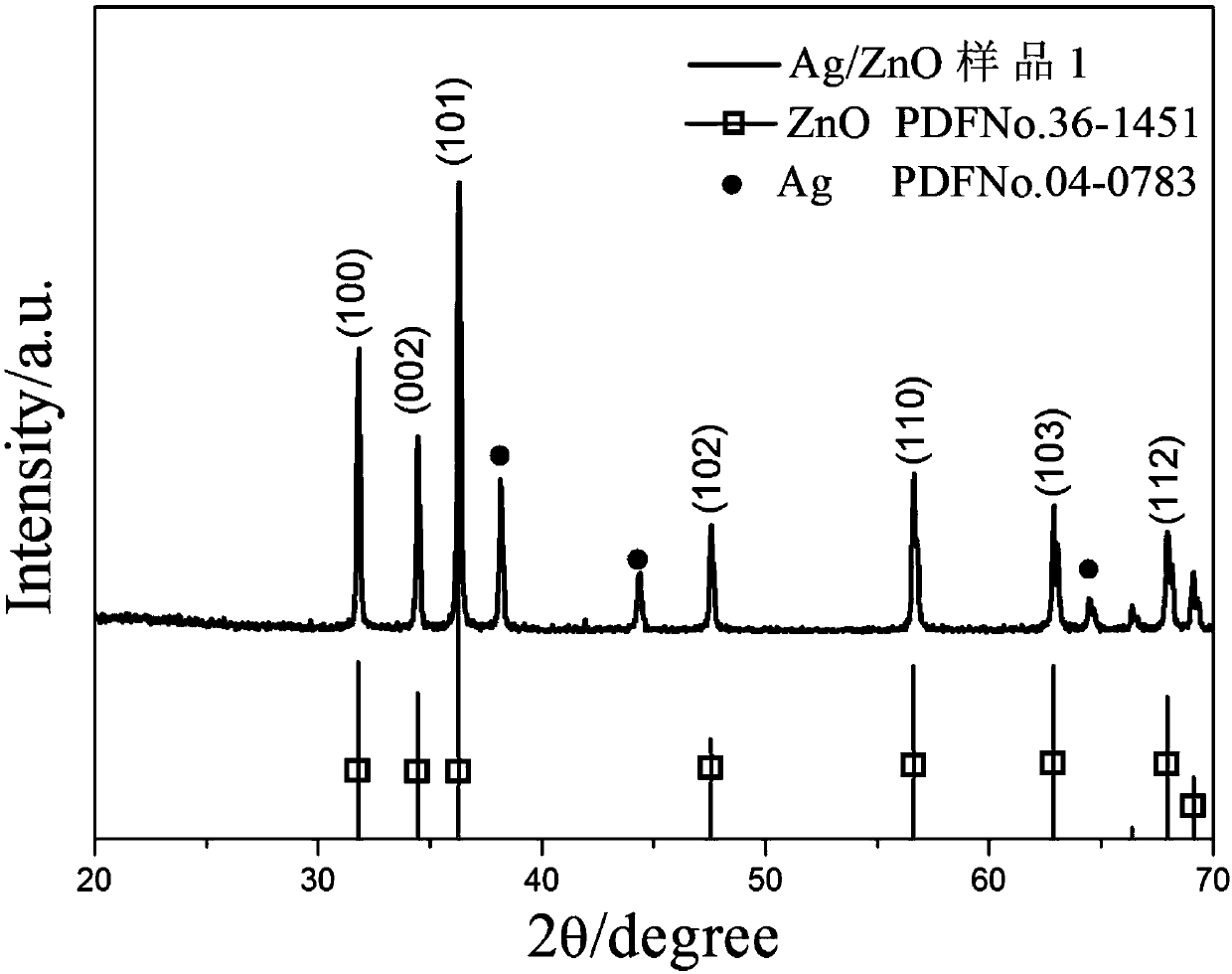
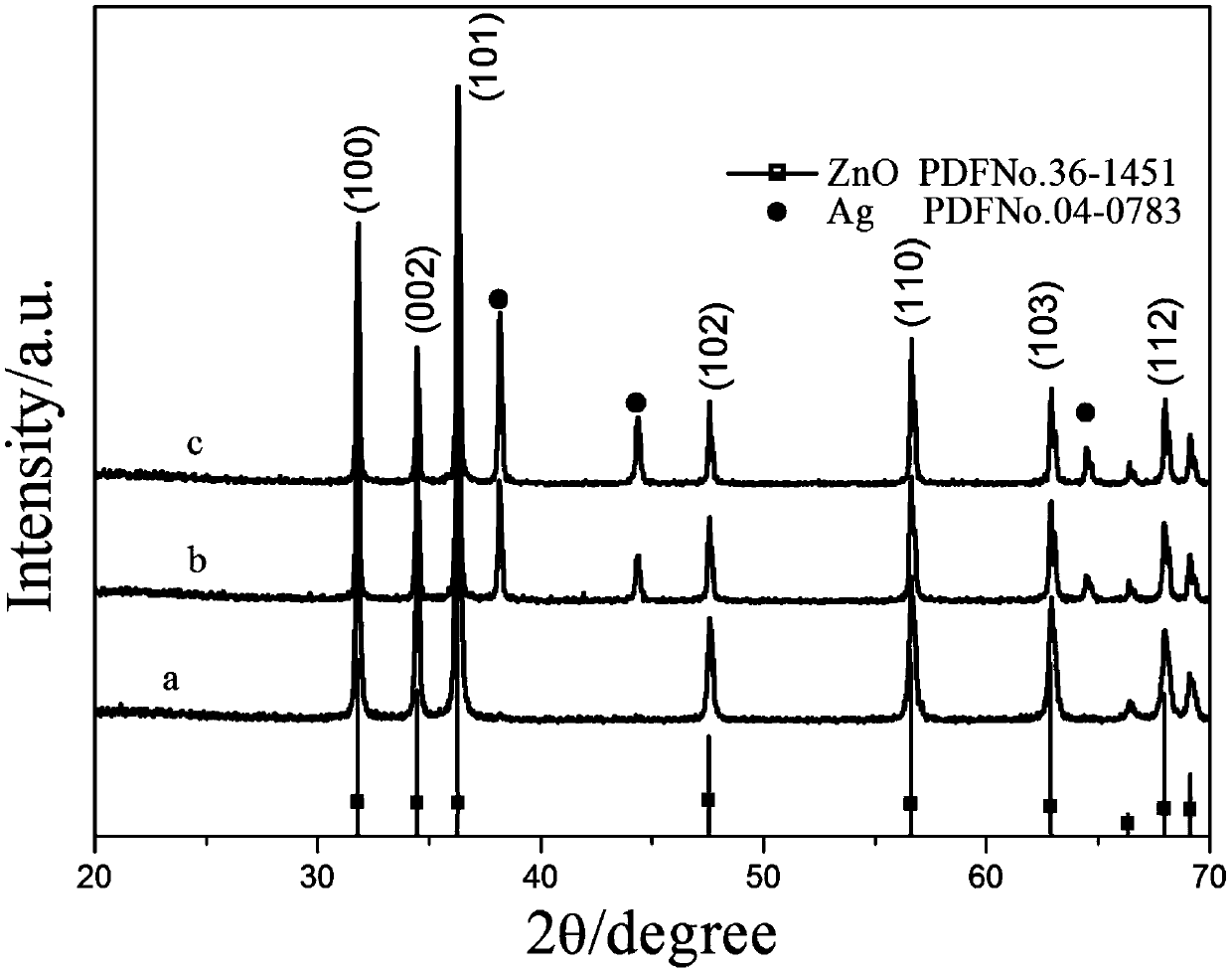
Image
Examples
Embodiment 1
[0021] Option 1: first add zinc acetate, L-aspartic acid, diethanolamine and deionized water into a 25mL reaction kettle and mix evenly, and then put them in an oven for reaction. After the reaction, the obtained product was washed twice with deionized water, and then washed twice with absolute ethanol, and then put into a vacuum drying oven to dry for 12 hours at a temperature of 40°C. After drying, ZnO powder was obtained.
[0022] Change the raw material components, hydrothermal reaction temperature, time, and make four groups of samples. See Table 1 for the data.
[0023] Table 1 Raw material ratio and reaction conditions of each group for ZnO sample preparation
[0024] Sample serial number
1
2
3
4
Zinc acetate (mmol)
1.8
2.0
2.5
2.0
L-Aspartic Acid (mg)
100
100
120
120
Diethanolamine (ml)
10
10
10
10
Deionized water (ml)
5
5
5
5
temperature(°C)
140
140
140
...
Embodiment 2
[0040] Photocatalytic experiment and ultraviolet-visible spectroscopy (uv-vis) analysis
[0041] Methyl orange (C 14 h 14 N 3 SO 3 Na), it can be used as an acid-base indicator, and its shape is orange-red powder, which is alkaline. Rhodamine B(C 28 h 31 CIN 2 o 3 ), is a kind of organic dye that is relatively difficult to degrade. Because it is toxic and has a certain risk of pollution to food and water sources, it is proposed to study the performance of the sample through the photocatalytic degradation experiment of rhodamine B and methyl orange. .
[0042] Weigh 40mg of ZnO sample or Ag / ZnO sample and mix with 10mL of methyl orange or rhodamine B solution, add 40mL of deionized water, stir on a magnetic stirrer for 60min in a dark environment, and then irradiate with a xenon lamp After 60 minutes, sample 2 mL with a straw at the beginning, and take a sample every 10 minutes. Then use the ultraviolet-visible spectrometer to measure the absorption curve of the sampl...
Embodiment 3
[0046] Reaction Mechanism and Product Photocatalytic Mechanism
[0047] 1. The growth mechanism of ZnO and Ag / ZnO
[0048] (1) In the hydrothermal reaction, ZnO will have the following reactions:
[0049] Zn 2+ +4OH - →Zn(OH) 4 2- (1)
[0050]
[0051] ZnO→ZnO X (1-X)V 0 , 0﹤X﹤1 (3)
[0052] Zinc acetate is used as the precursor of the product zinc oxide, and diethanolamine provides an alkaline environment to promote Zn(OH) 4 2- The formation of ZnO is to promote the production of ZnO precursor.
[0053] (2) The first few steps of the growth of Ag / ZnO are the same as the previous section, and the loading of Ag is completed on this basis:
[0054] Zn 2+ +4OH - →Zn(OH) 4 2- (1)
[0055]
[0056] ZnO→ZnO X (1-X)V 0 , 0﹤X﹤1 (3)
[0057] Ag + +2OH→Ag(OH) 2 - (4)
[0058] Zn(OH) 4 2- +2Ag(OH) 2 - → Ag 2 O / ZnO+2H 2 O+4OH - (5)
[0059] Ag 2 O / ZnO→Ag / ZnO X (1-X)V 0 , 0﹤X﹤1 (6)
[0060] Its growth process is basically similar to the previous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com