Organic blue light micromolecules based on anthracene-tetraphenylethylene and application of organic blue light micromulecules to preparation of non-doped organic electroluminescence device
An electroluminescent device, tetrastyrene technology, applied in the direction of electro-solid devices, electrical components, luminescent materials, etc., can solve the problems of low efficiency, device efficiency roll-off, etc., achieve simple synthesis, small efficiency roll-off, excellent electrical The effect of luminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
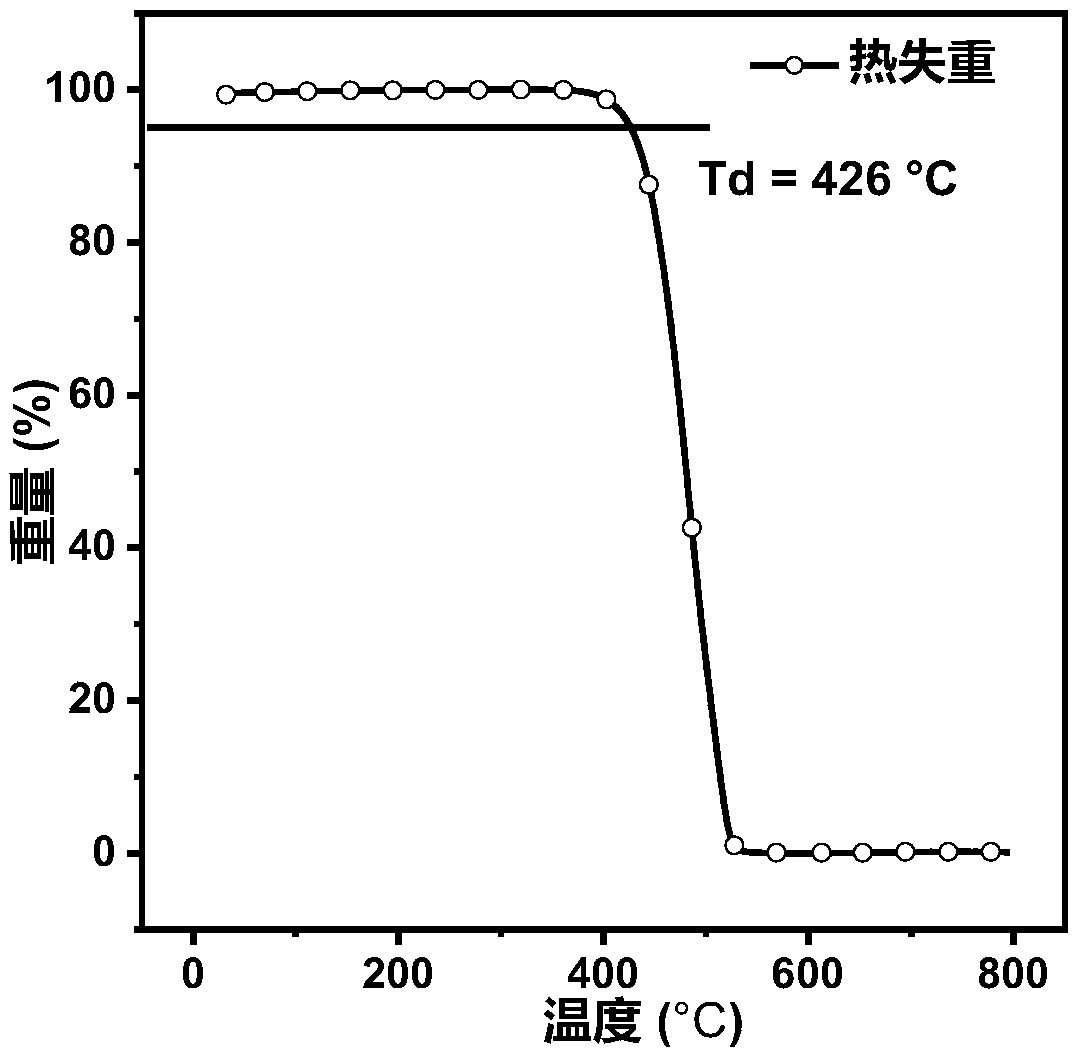
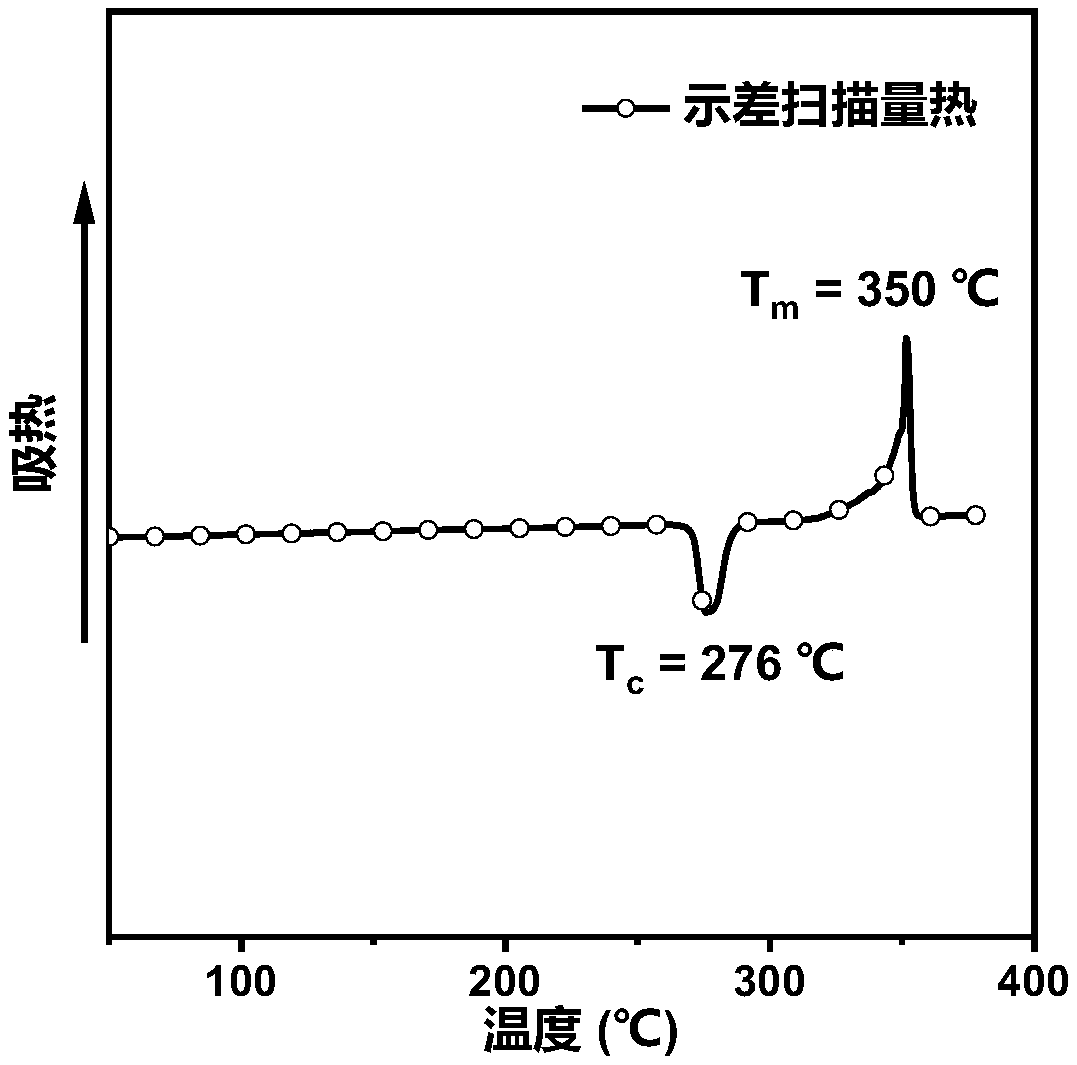
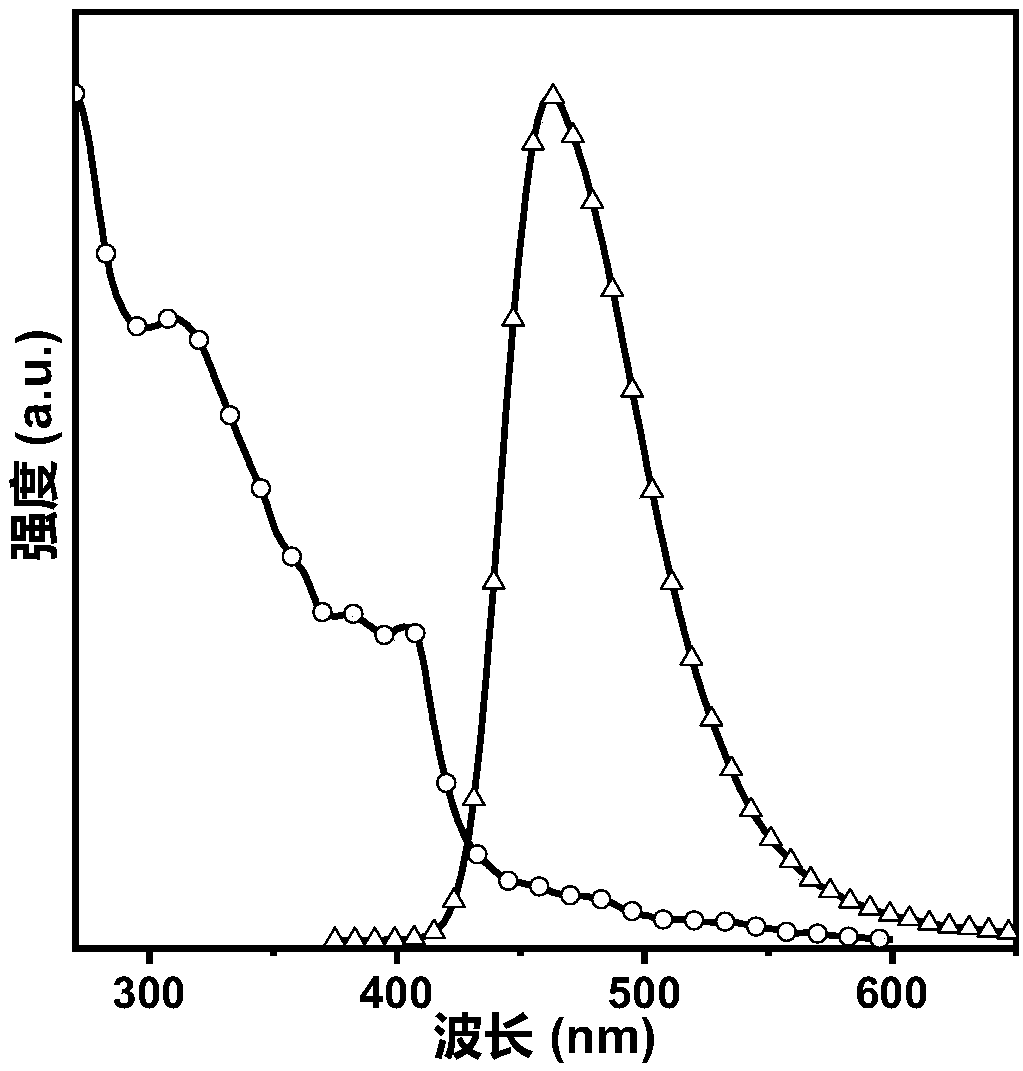
Image
Examples
Embodiment 1
[0030] Example 1: In the preparation of Example P1, the steps are as follows:
[0031] Synthesis of M1: Dissolve diphenylmethane (5.05mL, 30mmol) in 50mL of tetrahydrofuran, stir in an ice-water bath at 0°C, and add 13mL of n-butyllithium (2.4M, 30mmol) dropwise, in an ice-water bath at 0°C Stir for 30min. Then slowly add the mixed solution dropwise to the tetrahydrofuran solution containing 4-bromobenzophenone (6.50g, 25mmol), stir at room temperature for 6h, add saturated NH 4 Cl solution. The crude product was extracted with dichloromethane, and the extract was concentrated by rotary evaporation to obtain a white crude alcohol product. Dry the obtained crude alcohol product and dissolve it in 100mL of toluene, add p-toluenesulfonic acid (1.30g, 6.76mmol), reflux at 80℃ for 6h, use 10% NaHCO 3 The toluene layer was washed with the solution, extracted and separated, and concentrated to obtain a crude product, which was separated and purified by column chromatography with cycloh...
Embodiment 2
[0039] Example 2: Preparation of Example P2, the steps are as follows:
[0040] Synthesis of M4: M4 was synthesized by a one-pot method. In a 250mL round bottom flask, 9,10-phenanthrenequinone (6.24g, 30mmol), 4-bromobenzaldehyde (5.52g, 30mmol), aniline (14.3ML, 150mmol), ammonium acetate (9.24g, 120mmol) was dissolved in 150mL glacial acetic acid, and refluxed at 120°C for 4 hours. The reaction solution was poured into 100 mL of ice water, and a large amount of solids precipitated out instantly. Suction filtration, separation and purification by column chromatography (petroleum ether: dichloromethane = 1:1) to obtain a white solid (11.86 g, yield: 89%). MALDI-TOF (m / z)(M + ]: The measured value is 448.75 and the theoretical value is 448.06.
[0041]
[0042] Synthesis of M5: Dissolve M4 (4.48g, 10mmol) in 80mL freshly distilled tetrahydrofuran and place it in a low temperature reactor at -78℃ for 10min, freeze and degas three times, then slowly drop 6.2mL n-butyllithium (2.40M,...
Embodiment 3
[0046] Embodiment 3: The preparation of this embodiment P3, the steps are as follows:
[0047] Synthesis of P3: In a 100mL round-bottom flask, mix 4-(9H-carbazol-9-yl)phenylboronic acid (0.86g, 3mmol), M3(1.76g, 3mmol), tetrakistriphenylphosphine palladium (80mg, 0.070mmol), potassium carbonate (5.52g, 40mmol) was dissolved in 40mL toluene and 20mL aqueous solution, and refluxed at 90°C for 24 hours under nitrogen protection. The liquid was extracted and separated with dichloromethane, concentrated to obtain a crude product, which was separated and purified by column chromatography (petroleum ether: dichloromethane=2:1) to obtain a yellow solid (1.75 g, yield: 78%). 1 H NMR (500MHz, CD 2 Cl 2 , ppm, δ): 8.26(t,J=6.9Hz,2H),7.89(m,J=7.6,5.7Hz,4H),7.79–7.71(m,5H),7.58–7.53(m,2H), 7.51–7.43(m,4H),7.43–7.36(m,2H),7.34(d,J=8.1Hz, 2H),7.32–7.19(m,18H); mass spectrum MALDI-TOF(m / z)[M + ]: The measured value is 749.54 and the theoretical value is 749.96. Elemental analysis: C 58 H 39 T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com