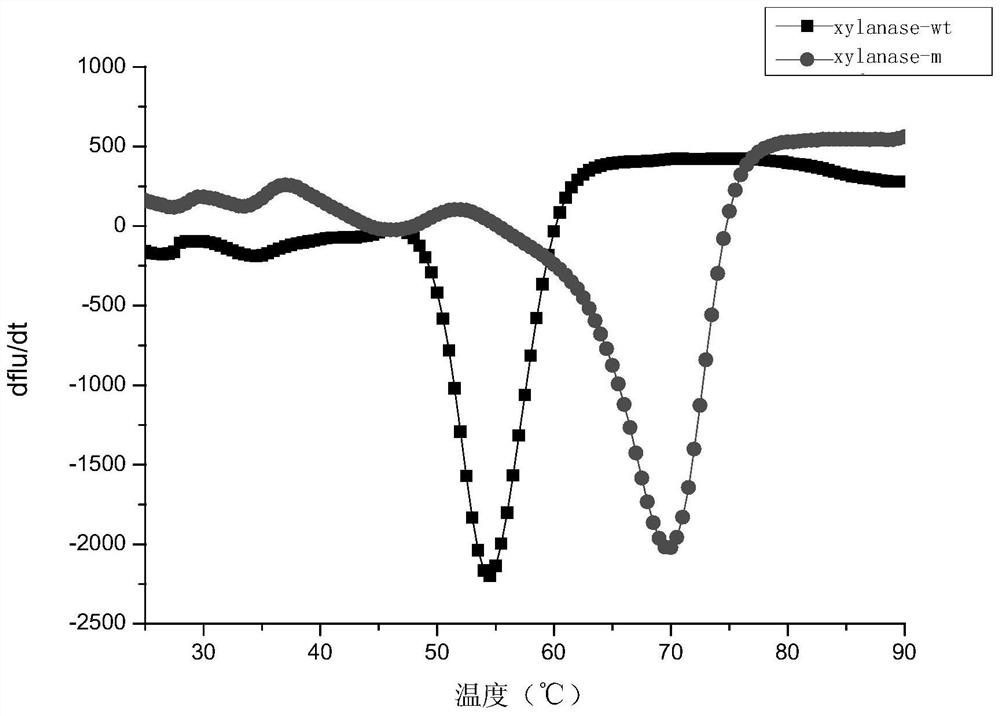
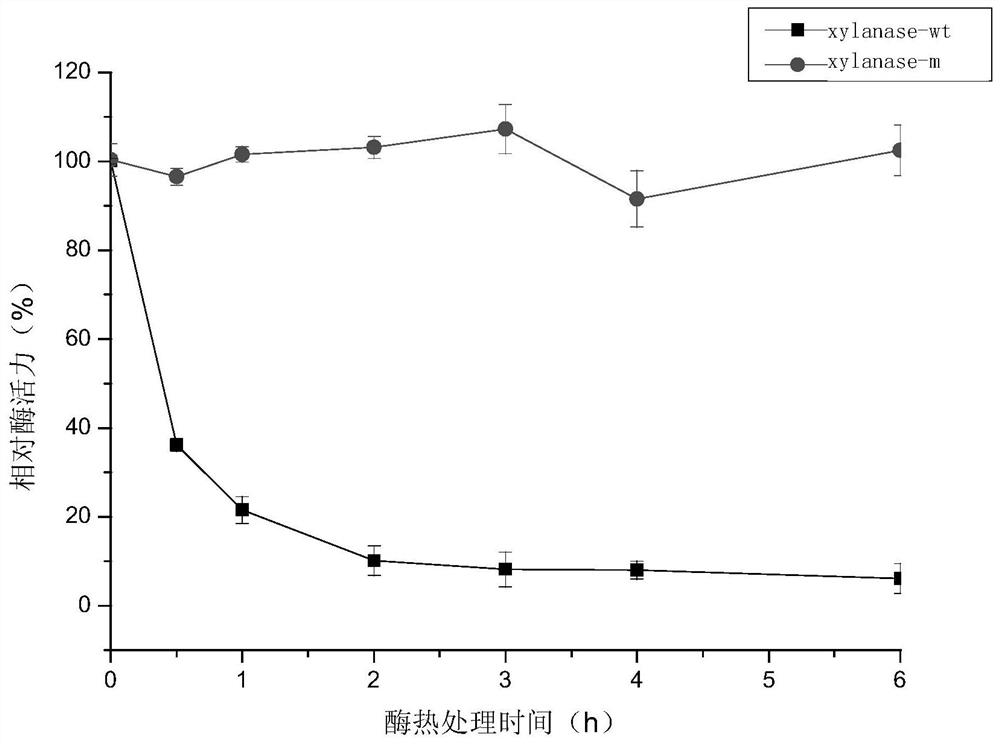
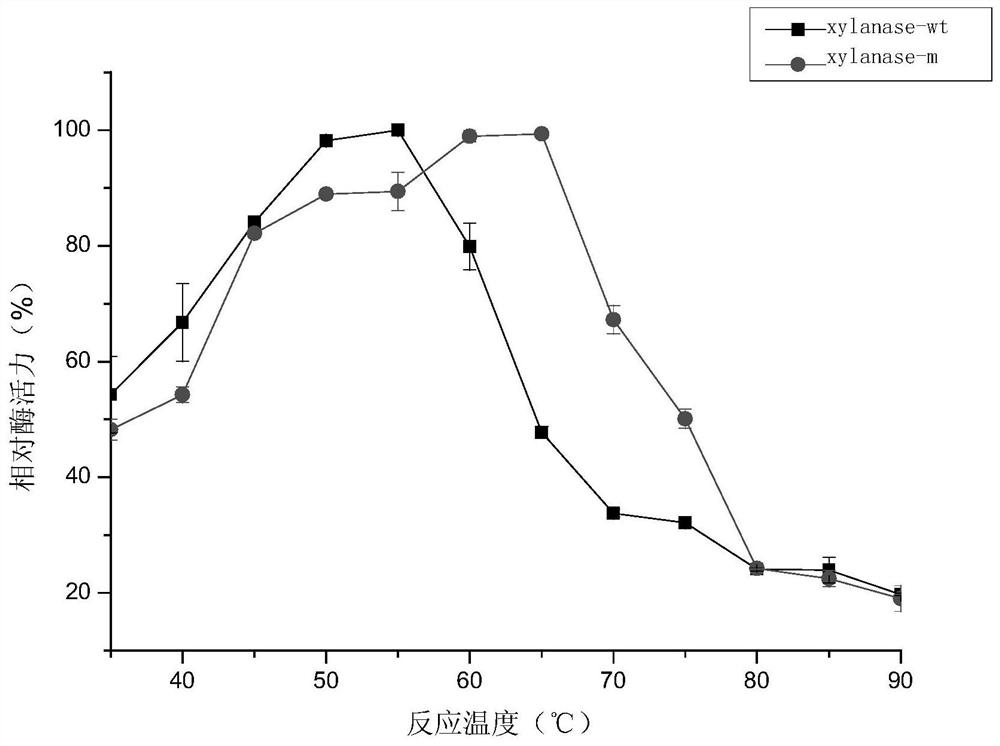
A kind of xylanase xylanase-m with high thermostability and its coding gene and application
A xylan and gene technology, applied in the field of genetic engineering, can solve the problems of large loss of activity and increased cost, and achieve the effects of improved thermal stability, improved practicability, and significant application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Construction of recombinant bacteria
[0037] Insert the double-stranded DNA molecule represented by nucleotide 5-708 of sequence 4 in the sequence list between the NcoI and EcoRI restriction sites of vector pET-28a(+) to obtain the recombinant plasmid pET28a-xylanase-wt. After sequencing, the structure of the recombinant plasmid pET28a-xylanase-wt is described as follows: the foreign DNA molecule is fused with part of the nucleotides on the vector backbone to form the fusion gene shown in sequence 4 of the sequence list, and the sequence shown in sequence 3 of the sequence list is expressed Protein shown. In sequence 3 of the sequence listing, the amino group of positions 5-10 constitutes his 6 Tag, amino acid residues 11-235 constitute wild-type xylanase. The recombinant plasmid pET28a-xylanase-wt was introduced into E. coli BL21 (DE3) to obtain a recombinant strain, which was named as recombinant strain BL21 / xylanase-wt.
[0038] Insert the double-stranded DN...
Embodiment 2
[0039] Example 2. Preparation of Xylanase
[0040] The test bacteria were: recombinant bacteria BL21 / xylanase-wt or recombinant bacteria BL21 / xylanase-m.
[0041] 1. Inoculate the test bacteria into liquid LB medium, and cultivate to OD at 37℃ and 250rpm with shaking 600nm The value is 1.
[0042] 2. After completing step 1, add IPTG to the system and make the concentration of 0.1mM, 30℃, 200rpm shaking culture for 16h.
[0043] 3. After completing step 2, collect the bacteria by centrifugation, resuspend in pH7.0, 50mM PBS buffer, and ultrasonically disrupt (parameters of ultrasonic disruption: power 30%, total time 20min, stop for 5s every 5s of work), then 12000rpm Centrifuge for 10 min and collect the supernatant.
[0044] 4. Take the supernatant obtained in step 3 and perform affinity chromatography on a nickel column.
[0045] Nickel column (column volume is 2mL): 6FF Ni Sepharose (Beijing Jiangchen Yuanyuan Biotechnology Co., Ltd.; catalog number: HA-0710-02).
[0046] Washing so...
Embodiment 3
[0053] Example 3 Activity analysis of xylanase (DNS method)
[0054] One unit (U) of enzyme activity is defined as the amount of enzyme that releases 1 μmol of reducing sugar per minute under given conditions.
[0055] 1. Prepare the solution to be tested.
[0056] Take the xylanase-wt solution or xylanase-m solution prepared in Example 2, and dilute it with a pH 7.0, 50 mM PBS buffer as the test solution.
[0057] 2. Detect the protein concentration of the solution to be tested.
[0058] Detect the concentration of the target protein in the test solution prepared in step 1 (calculated as the total protein concentration).
[0059] 3. Detect the enzyme activity of the solution to be tested.
[0060] 1. Prepare the initial reaction system.
[0061] The initial reaction system (100μL) consists of the test solution, birchwood xylan and pH7.0, 50mM PBS buffer. In the initial reaction system, the concentration of protein is 0.005mg / mL, and the concentration of birchwood xylan is 1g / 100mL.
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com