Preparation method of decellularized derma matrix of human source
A decellularized dermis and decellularized technology, applied in the field of medical materials, can solve the problems of complex process, complicated steps, and long cycle, and achieve the effect of small immune rejection, simple and reliable production process, and high biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of human-derived acellular dermal matrix, the specific steps are as follows:
[0038] (1) Take the stored human skin;
[0039] (2) Remove subcutaneous fat components;
[0040] (3) Step (2) removes the subcutaneous skin with fatty components, cleans and disinfects with 2% iodophor, deiodines with 75% alcohol, and then repeatedly soaks and cleans the skin with sterile distilled water;
[0041] (4) Treat the skin after step (3) washing with 2.5mg / ml Dispase II solution overnight;
[0042] (5) Discard the Dispase II solution, remove the epidermis, and then wash with physiological saline to obtain the dermis;
[0043] (6) the dermis that step (5) obtains contains Ca with 2mol / L 2+ NaCl solution for 18h, Ca 2+ The final concentration is 5mM / L;
[0044] (7) After discarding the 2mol / L sodium chloride solution, wash with normal saline;
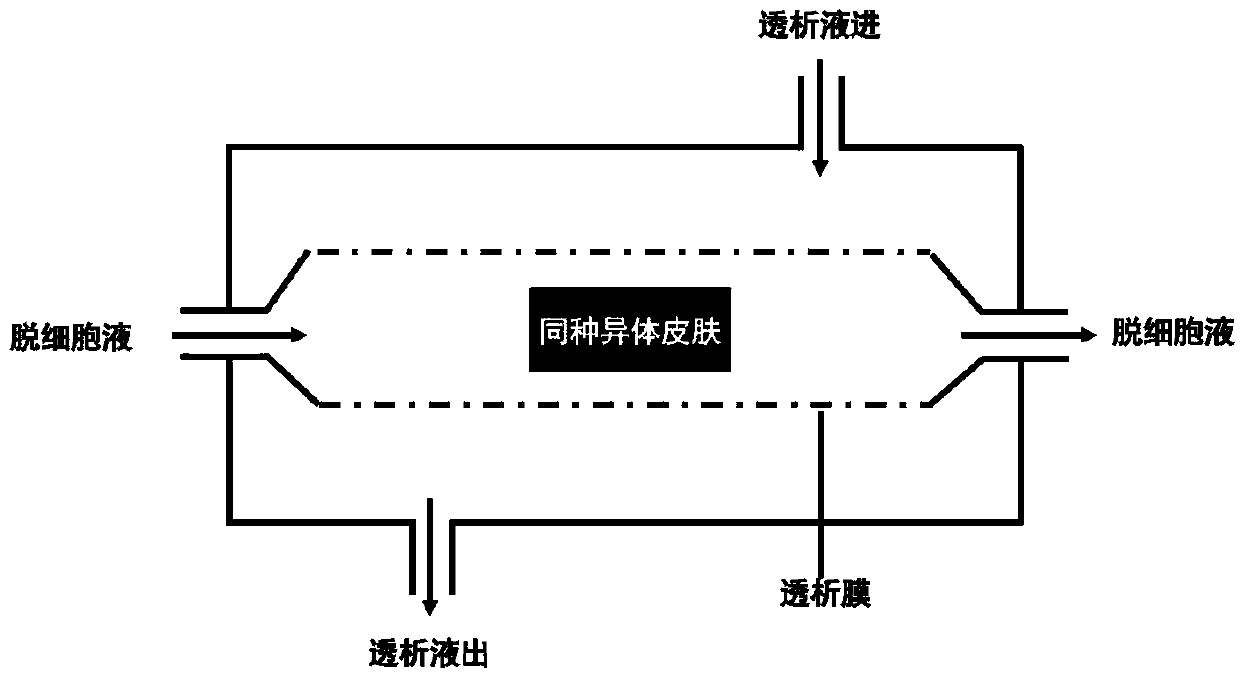
[0045] (8) Place the dermis cleaned in step (7) in the prepared "decellularized solution", which contains the followi...
Embodiment 2
[0048] Example 2 Internal Structure of Acellular Dermal Matrix
[0049] (1) Cut the acellular dermal matrix obtained above into a size of 1 cm × 1 cm, and paste it on a metal tray; the section of the acellular dermal matrix is quenched with liquid nitrogen to make it hard and brittle, and then broken; the section faces upward Adhesive to the metal tray.
[0050] (2) After spraying gold in the sample preparation room of the electron microscope, it was put on the machine, observed and photographed by the scanning electron microscope, and the results were as follows: image 3 shown.
[0051] The results showed that there were no cell residues in the tissue, and the collagen structure was complete, similar to normal skin.
Embodiment 3
[0052] Example 3 HE staining of acellular dermal matrix
[0053] (1) Fix the normal skin tissue and the acellular dermal matrix in 4% paraformaldehyde fixative solution for 24 hours respectively;
[0054] (2) Alcohol dehydration, xylene transparency;
[0055] (3) Dip in wax for 30 minutes, embed, and make sagittal paraffin sections with a thickness of 5 μm;
[0056] (4) Gradient alcohol carries out dewaxing, dehydration, HE staining;
[0057] (5) Transparent, sealed, observed under a light microscope, the results of HE staining of normal skin tissue are as follows: Figure 4 As shown, the results of HE staining of acellular dermal matrix are as follows Figure 5 shown.
[0058] The results showed that there were no cell residues in the skin tissue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com