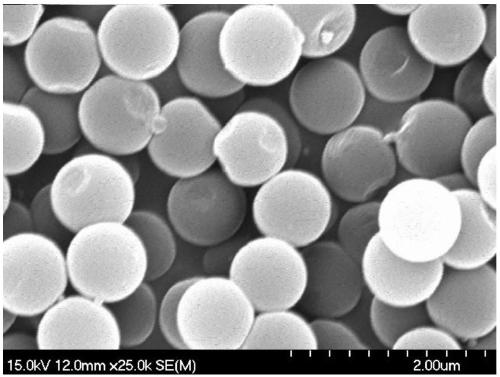
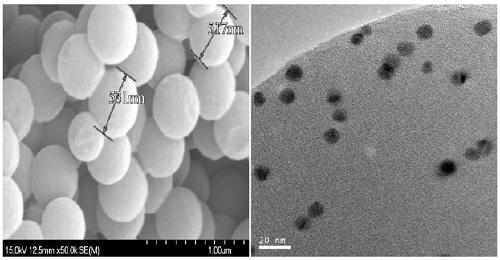
Sulfur carbon ball loaded precious metal catalyst and preparation method and application thereof
A noble metal catalyst and catalyst technology, which are applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of the instability of carbon-supported noble metal catalysts, the unsatisfactory effect of doping sulfur, and the need to improve the selectivity, etc. problems, to achieve the effect of improving metal utilization, improving material mass transfer efficiency, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Take 100ml of deionized water in a three-necked flask, install a condensation reflux device in one port, and seal the other two ports with glass stoppers; then add 0.5g cetyltrimethylammonium bromide and 1.5g cysteine solid, set The temperature of the water bath was 30°C, and magnetic stirring was started for 1 h until the solid was completely dissolved. Subsequently, add 5 g of 4,4'-thiodisalicylic acid thiophenol solid, continue to stir for 0.5 h until the solid is completely dissolved, then open one of the glass stoppers, and slowly add 2 ml of concentrated H 2 SO 4 The solution is in the above mixed solution, and the rate of addition is strictly controlled to prevent the temperature rise caused by the heat of dissolution from exceeding 2°C. Continue magnetic stirring, from room temperature to 60°C at a heating rate of 0.1°C / min, keep for 6h, then continue to rise to 90°C at a heating rate of 0.1°C / min, keep for 6h, and finally continue to rise to boiling at a hea...
Embodiment 2
[0052] Take 100ml of deionized water in a three-necked flask, install a condensation reflux device in one port, and seal the other two ports with glass stoppers; then add 1g of cetyltrimethylammonium bromide and 2g of cysteine solid, and set the temperature of the water bath At 30°C, start magnetic stirring for 1 h until the solid is completely dissolved. Subsequently, 8 g of 4,4'-thiodisalicylic acid thiophenol solid was added, and stirring was continued for 0.5 h until the solid was completely dissolved, then one of the glass stoppers was opened, and 2 ml of concentrated H 2 SO 4 The solution is in the above mixed solution, and the rate of addition is strictly controlled to prevent the temperature rise caused by the heat of dissolution from exceeding 2°C. Continue magnetic stirring, from room temperature to 60°C at a heating rate of 0.1°C / min, keep for 8h, then continue to rise to 90°C at a heating rate of 0.1°C / min, keep for 8h, and finally continue to rise at a heating ...
Embodiment 3
[0056]Take 100ml of deionized water in a three-necked flask, install a condensation reflux device in one port, and seal the other two ports with glass stoppers; then add 1g of cetyltrimethylammonium bromide and 2g of cysteine solid, and set the temperature of the water bath At 30°C, start magnetic stirring for 1 h until the solid is completely dissolved. Subsequently, add 10 g of 4,4'-thiodisalicylic acid thiophenol solid, continue to stir for 0.5 h until the solid is completely dissolved, then open one of the glass stoppers, and slowly add 2 ml of concentrated H 2 SO 4 The solution is in the above mixed solution, and the rate of addition is strictly controlled to prevent the temperature rise caused by the heat of dissolution from exceeding 2°C. Continue magnetic stirring, raise the temperature from room temperature to 60°C at a rate of 1°C / min, keep for 12 hours, then continue to increase the rate of temperature at 1°C / min to 90°C, keep for 12 hours, and finally continue t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com