Synthesis method of aryl-substituted homoallylic alcohol
A high allyl alcohol, synthetic method technology, applied in the formation/introduction of hydroxyl groups, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of harsh reaction conditions, limited raw material sources, expensive catalysts, etc., and achieve the reaction route The effect of short time, wide source of raw materials and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
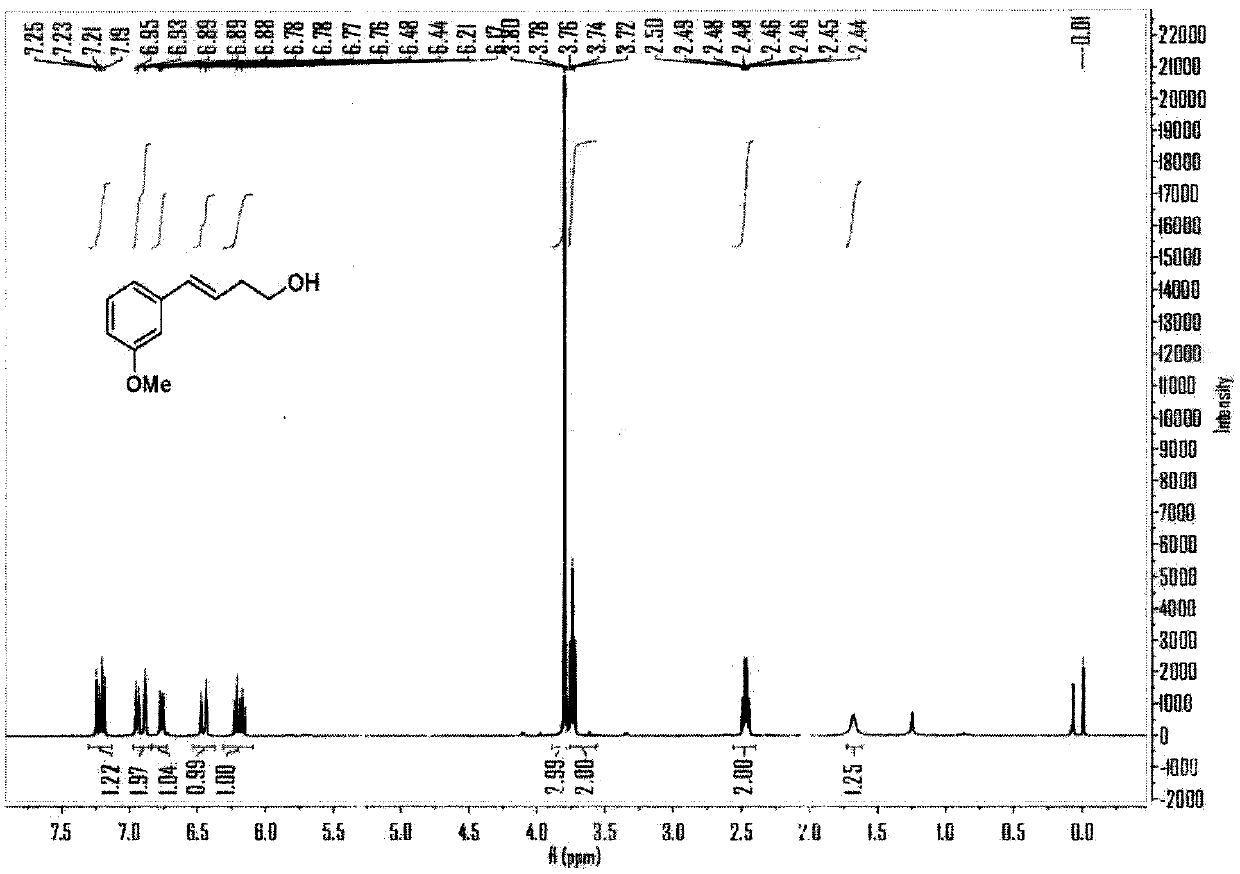
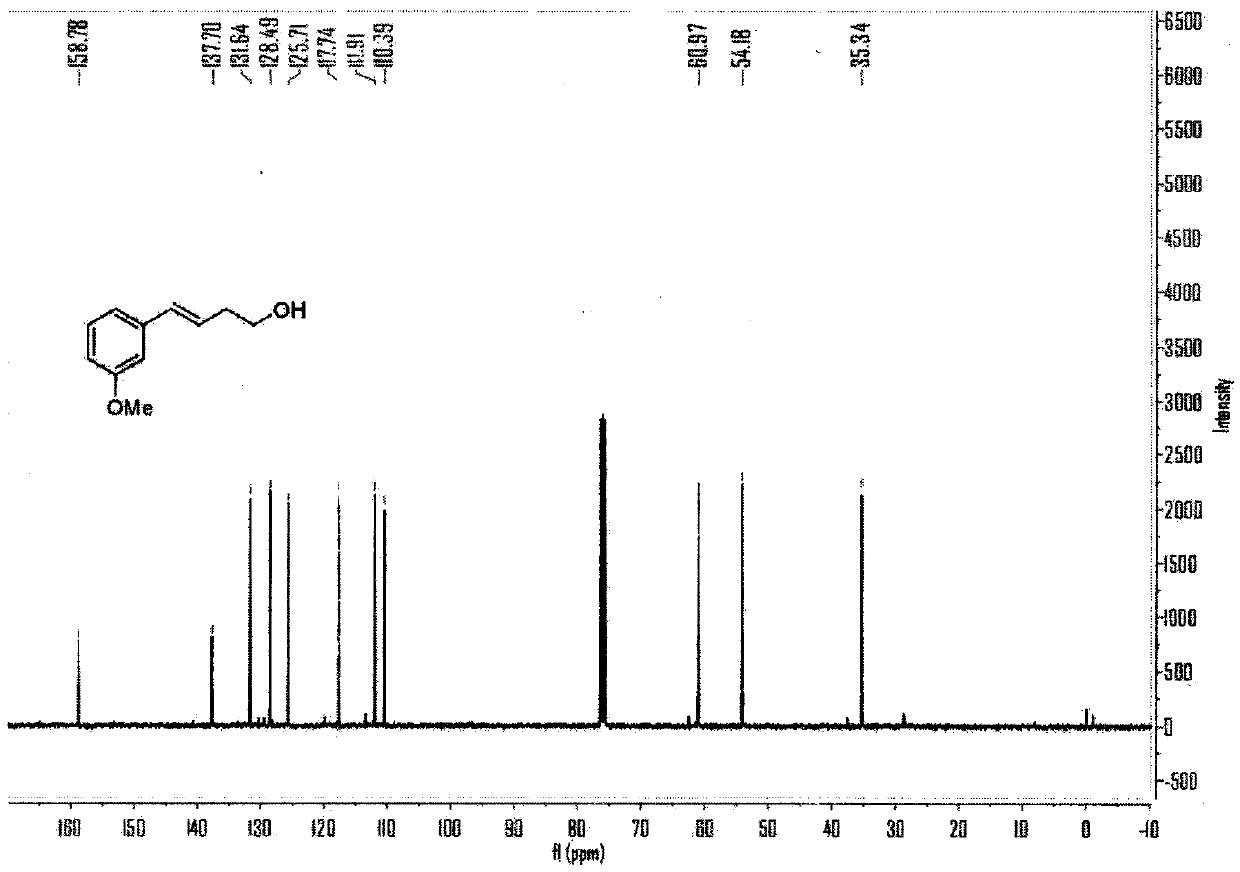
[0061] Embodiment 1, the reaction formula of this embodiment is as follows:
[0062]
[0063] (1) Under air, cuprous chloride (CuCl, 10mol%), tetramethylethylenediamine (TMEDA, 15mol%), lithium tert-butoxide (LiO t Bu, 2.5eq), m-methoxy-aryl neopentyl glycol boroester (2eq) were added to a sealed reaction tube with a branch tube containing magnetons, and the reaction tube was flushed with argon three times. Under the protection of argon, add 0.6mL N,N-dimethylformamide to the reaction tube, then add vinyl epoxy (0.25mmol) to the reaction solution under the protection of argon, stopper the stopper, and place at 60 °C in an oil bath and stirred for 10 hours.
[0064] (2) Quench the material obtained in step (1) by adding saturated ammonium chloride, add ethyl acetate and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0065] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product...
Embodiment 2
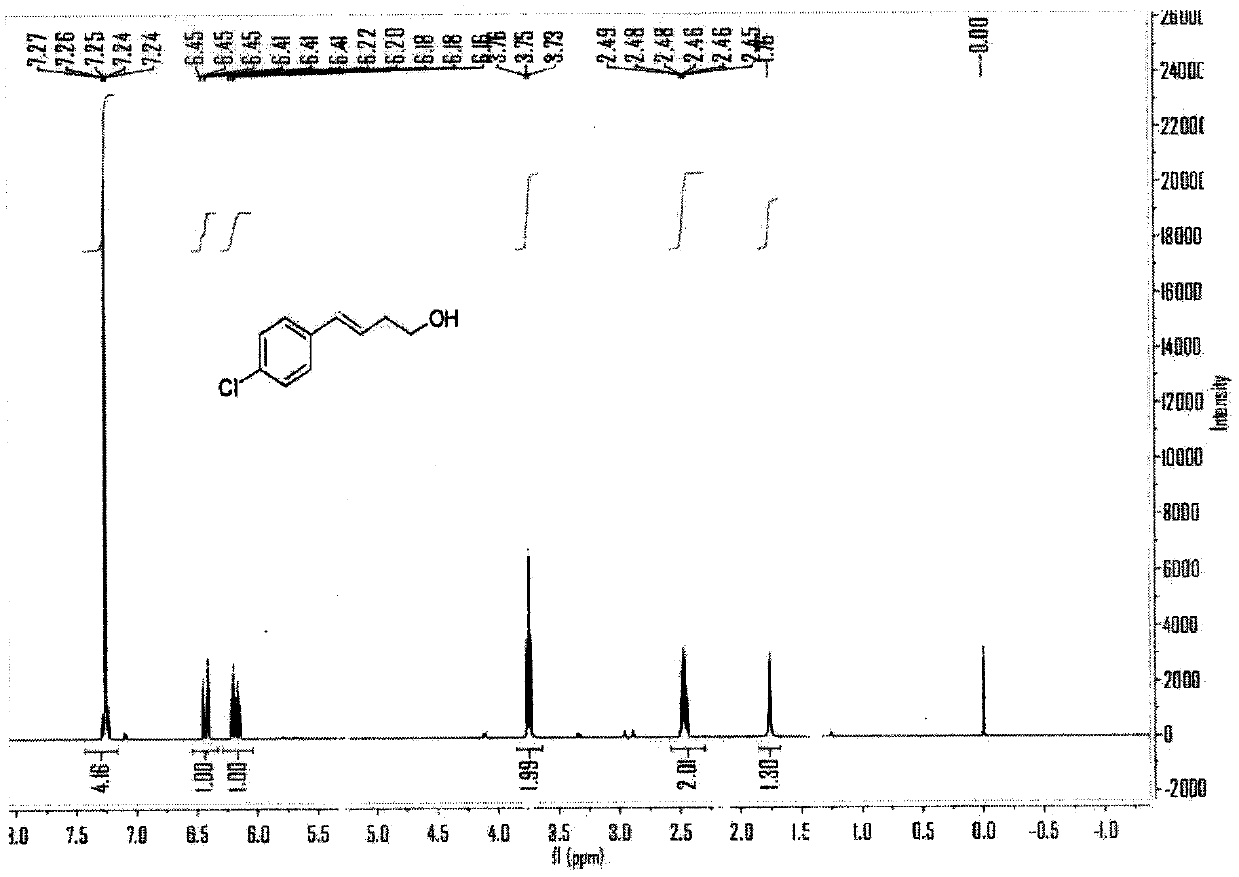
[0067] The reaction formula of this embodiment is as follows:
[0068]
[0069] (1) Under air, cuprous chloride (CuCl, 10mol%), tetramethylethylenediamine (TMEDA, 15mol%), lithium tert-butoxide (LiO t Bu, 2.5eq), p-chloroaryl neopentyl glycol boroester (2eq) were added to a sealed reaction tube with a branch tube containing magnetons, and the reaction tube was flushed with argon three times. Under the protection of argon, add 0.6mL N,N-dimethylformamide to the reaction tube, then add vinyl epoxy (0.25mmol) to the reaction solution under the protection of argon, stopper the stopper, and place at 60 °C in an oil bath and stirred for 10 hours.
[0070] (2) Quench the material obtained in step (1) by adding saturated ammonium chloride, add ethyl acetate and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0071] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purif...
Embodiment 3
[0073] The reaction formula of this embodiment is as follows:
[0074]
[0075] (1) Under air, cuprous chloride (CuCl, 10mol%), tetramethylethylenediamine (TMEDA, 15mol%), lithium tert-butoxide (LiO t Bu, 2.5eq), p-bromoaryl neopentyl glycol boroester (2eq) were added to a sealed reaction tube with a branch tube containing magnetons, and the reaction tube was flushed with argon three times. Under the protection of argon, add 0.6mL N,N-dimethylformamide to the reaction tube, then add vinyl epoxy (0.25mmol) to the reaction solution under the protection of argon, stopper the stopper, and place at 60 °C in an oil bath and stirred for 10 hours.
[0076] (2) Quench the material obtained in step (1) by adding saturated ammonium chloride, add ethyl acetate and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0077] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purify...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com