Mercury ion detection probe, and preparation method and application thereof
A technology for detecting probes and mercury ions, applied in chemical instruments and methods, fluorescence/phosphorescence, organic chemistry, etc., can solve the problems of expensive instruments and troublesome sample processing, and achieve low detection limit, sensitive recognition, and strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of fluorescent probe of the present invention
[0043] The preparation method of the fluorescent probe of the present invention is as follows:
[0044]
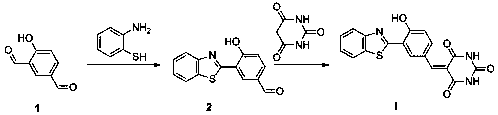
[0045] (1) Preparation of intermediate compound 2
[0046] Take compound 1 and o-aminothiophenol dissolved in dimethyl sulfoxide (DMSO), the ratio of the amount of the compound 1 and o-aminothiophenol is 1:1, heated at 60 degrees for 6 hours, TLC monitoring During the reaction process, when the reaction is complete, the reactants are cooled to room temperature, water is added, and then separated and extracted, dried, and then subjected to column chromatography to obtain compound 2;
[0047] (2) Preparation of fluorescent probe I
[0048] Dissolve compound 2 and barbituric acid in DMSO. The ratio of the amount of compound 1 to barbituric acid is 1:1. Heat and stir at 70°C for 10 hours. The solid product in the reaction solution is filtered and separated. Washed with anhydrous ethanol three times to obtain a yellow sol...
Embodiment 2
[0051] Ratio Fluorescence Probe Molecular Working Curve
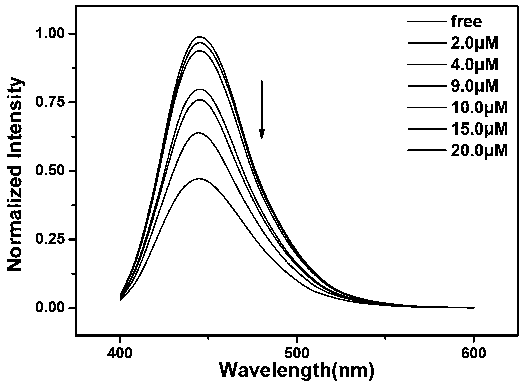
[0052] Take 2 mL of the prepared aqueous dispersion solution of fluorescent probe molecules and add 20 μL of different concentrations (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 50 μmol / L) Mercury iodide aqueous solution, after 60 min, the fluorescence spectrometer records the change of fluorescence intensity at 455nm. Take the fluorescence intensity as the ordinate and the copper ion concentration as the abscissa to plot, and fit the working curve of the ratio fluorescent probe molecule. In this example, the ratio fluorescent probe is used to determine the concentration of copper ions. Under the excitation of 375nm excitation light, the relationship between the fluorescence intensity and the concentration of copper ions is as follows: figure 1 As shown, the value of fluorescence intensity decreases with increasing mercury ion concentration.
Embodiment 3
[0054] Fluorescent probe molecular cation selective determination
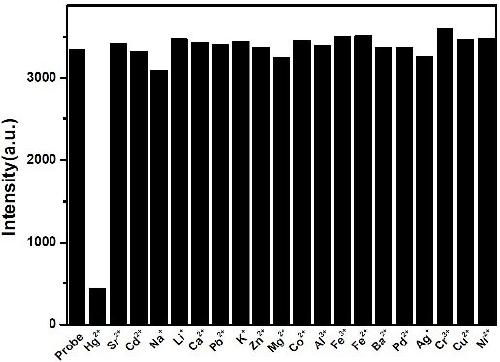
[0055] Take 2 mL of the aqueous dispersion solution of the ratio fluorescent probe molecules prepared in Example 2, and add 20 μL of 0.01M Li + , K + , Na + , Sr 2+ , Ba 2+ , Mg 2+ , Ca 2+ , Zn 2+ , Ni 2+ , Co 2+ , Hg 2+ , Pb 2+ , Pd 2+ , Fe 2+ , Mn 2+ , Al 3+ , Cr 3+ , Cd 2+ , Fe 3+ After the aqueous solution is left for 60 minutes, the fluorescence spectrometer records the change in fluorescence intensity at 445 nm. Experimental results show that, except for mercury ions, other ions did not cause a significant change in fluorescence at 445 nm, indicating that the probe molecule of the present invention has good selectivity. In this example, the fluorescent probe molecules are selectively determined. Under the excitation of 375nm excitation light, the relationship between the fluorescence intensity and the changes of different ions is shown in figure 2 Shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com