Ceramide analog b and its preparation method and application
A technology for ceramides and analogs, applied in the field of medicine, can solve the problems of biological application obstacles, the activity needs to be improved, the bottleneck in the chemical synthesis of ceramides, etc., and achieves the effects of low performance measurement, inhibition of tumor cell proliferation, and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
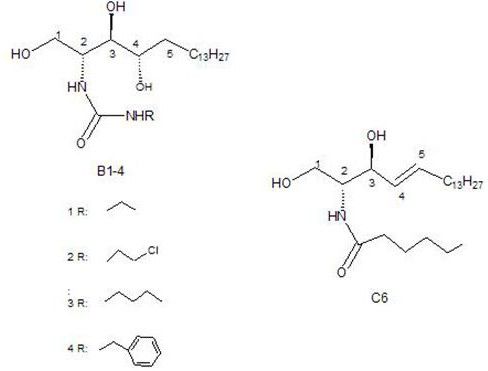
[0044] Synthesis of New Ceramide Analog B
[0045] 1) 1 g of (2S,3R,4R)-2-amino-1,3,4-octadecanetriol (for the preparation of sphingosine B, see APPLIED ANDENVIRONMENTAL MICROBIOLOGY, 2003, 69(2): 812–819) dissolved in Dissolve in 60 ml of chloroform at 40°C. After cooling down to room temperature, add 0.432 g of isocyanate-2-chloroethyl ester dropwise, stir at room temperature, track the reaction by thin layer chromatography, and end the experiment after the reaction raw material sphingosine disappears. , concentrated by rotary evaporation, and subjected to silica gel column chromatography with a mobile phase of chloroform / methanol=20:1 to obtain 0.959 g of a new ceramide analogue (B2), a white solid, with a yield of 72.3%. The structure was identified by NMR and mass spectrometry. 1 H NMR (400 MHz, C 5 D. 5 N) δ 4.94 (s, 1H), 4.47 (d, J = 20.9 Hz, 2H), 4.28 (s,2H), 3.77 (s, 3H), 2.16 (s, 1H), 1.89 (s, 1H), 1.67 (s, 1H), 1.33 (d, J =65.1 Hz, 20H), 0.87 (t, 3H). 13 ...
Embodiment 2
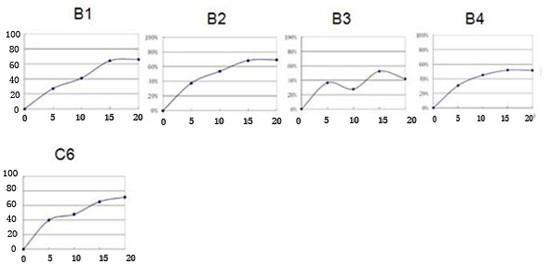
[0057] The new ceramide analogue B inhibits the proliferation of human pancreatic cancer cells and human intestinal cancer cells.
[0058] All human cancer cells used in the biological experiments are colorectal cancer cells LS174T, colon cancer cells SW480, and colon cancer cells SW620; pancreatic cancer cells in situ BxPC-3, pancreatic cancer cells PANC-1, and pancreatic cancer cells SW1990. The culture system was DMEM medium containing 10% fetal bovine serum, and then placed at 37°C, 5% CO 2 , cultured under saturated humidity. Containing new ceramide analogues B (B1, B2, B3, B4) and control C6 (purchased) were prepared with DMSO into 5μM, 10μM, 15μM, 20μM stock solutions, all samples need to be fully dissolved.
[0059] (1) Select tumor cells in the logarithmic growth phase to make a single cell suspension, inoculate them in different 96-well flat culture plates, adjust the number of cells to be consistent, the number of cells in each well is 3000, and the suspension is 2...
Embodiment 3
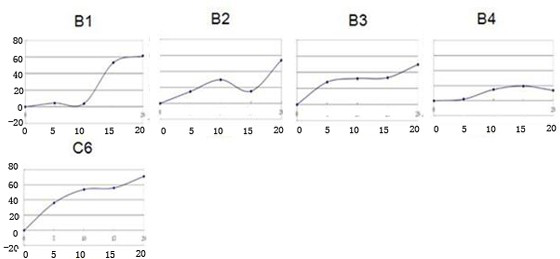
[0068]The new ceramide analogue B2 inhibits the proliferation of human chronic myelogenous leukemia cell K562 and human monocytic leukemia cell SHI-1.
[0069] Culture of leukemia cell lines
[0070] K562 and SHI-1 leukemia cells were inoculated in IMDM medium containing 10% and 15% newborn bovine serum and 100 U / L of penicillin and streptomycin, respectively, and placed at 37°C and 5% CO 2 Suspension culture in an incubator with saturated humidity, the medium was changed every 2-3 days, and the cells in the logarithmic growth phase were taken during the experiment.
[0071] Treating Leukemia Cells
[0072] Take the K562 and SHI-1 leukemia cells in the logarithmic growth phase respectively, and adjust the cell concentration to 10 5 / ml, the final concentrations of B2 and C6 were 10mg / L, and they were inoculated in culture flasks respectively. The above culture system was placed at 37°C, 5% CO 2 and saturated humidity incubator, and change the liquid every other day. Take ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com