Gefitinib preparation method
A technology of gefitinib and compounds, which is applied in the field of preparation of gefitinib, can solve the problems of many steps of side reactions, many by-products, unprotected hydroxyl groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of formula II compound:
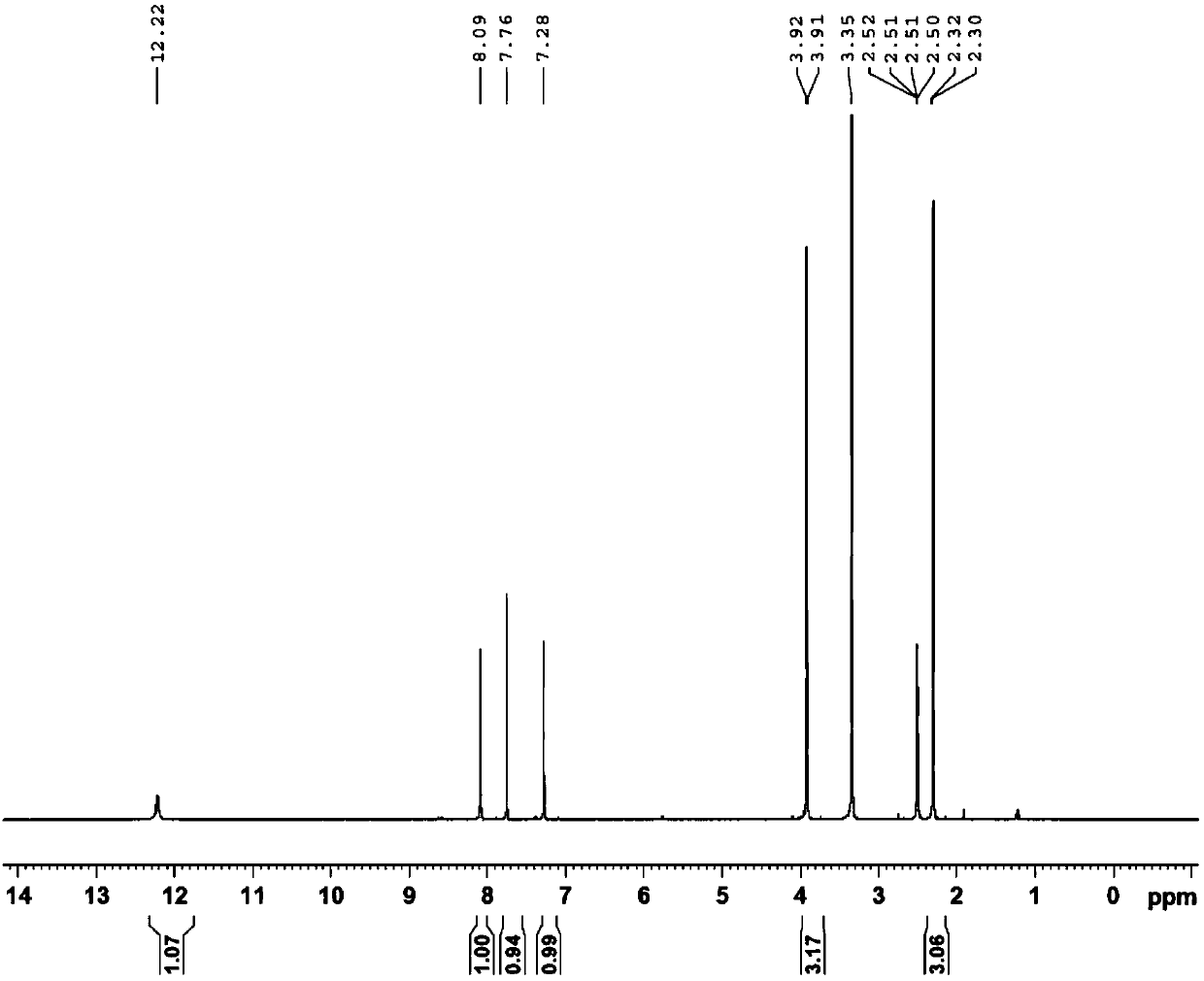
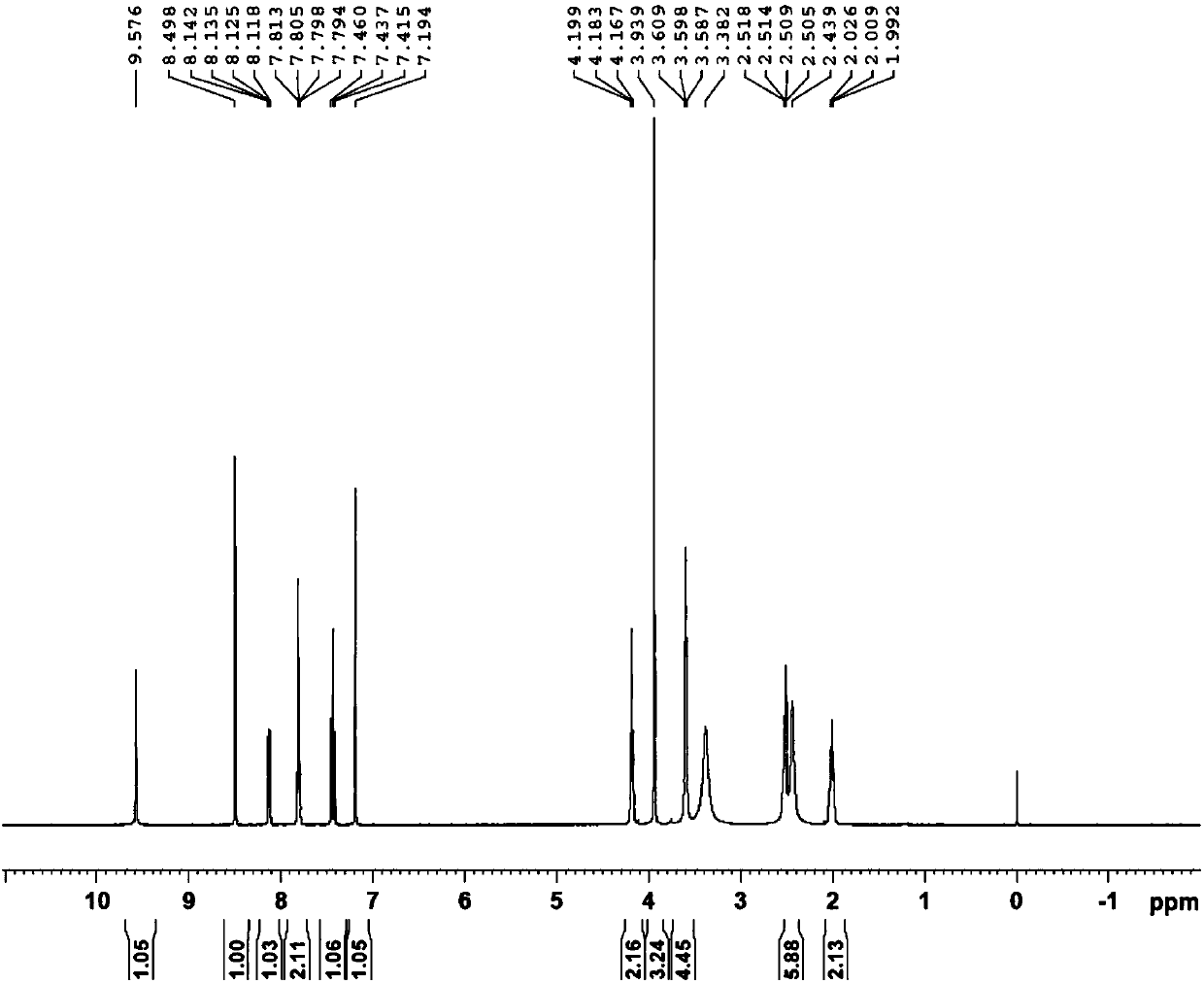
[0094] Add 200g (1.2mol) of 3,4-dimethoxybenzaldehyde into a 2L three-necked flask, add 1L of dichloroethane, stir until completely dissolved at 25°C, add 200mL (2.93mol) of 65% nitric acid dropwise into the reaction flask, Control the dropping temperature between 25-40°C, and the dropping is completed in about 1 hour. Stirring was continued at 35°C for 6 hours, and the reaction was monitored by HPLC to complete. Add water (1.5 L) for extraction, collect the organic phase, wash once with saturated sodium bicarbonate solution, and once with saturated brine, dry the organic phase with anhydrous sodium sulfate, filter the organic phase and concentrate under reduced pressure to obtain the crude compound of formula II, use After beating with n-hexane, 226.2 g of the yellow compound of formula II was obtained by filtration, with a molar yield of 89% and a purity of 98%. 1 H-NMR (CDCl 3 , 400MHz) δ4.04(3H, s), 4.05(3H, s), 7.42(1H, ...
Embodiment 2
[0096] The preparation of formula III compound:
[0097] Add 370 g (1.75 mol) of the compound of formula II into a 5 L three-necked flask, add 1.75 L of acetonitrile, 0.7 L of water, and 64.4 g (0.47 mol) of potassium dihydrogen phosphate, and stir to obtain a cloudy solution. 2.38 kg (2.63 mol) of 10% sodium chlorite solution was added dropwise, the temperature of the addition was controlled at 25-35° C., the stirring was continued for 1 hour after the addition was completed, and the reaction was monitored by HPLC. 36% hydrochloric acid was added dropwise until the pH was about 2, a large amount of solids were precipitated, the solids were collected by filtration, and dried at 50° C. to obtain 350.5 g of a yellow compound of formula III with a molar yield of 88% and a purity of 97%. 1 H-NMR (DMSO-d 6 , 400MHz) δ3.83(6H, s), 7.72(1H, s), 7.90(1H, s), 11.00(1H, s); MS(ESI, m / z): 228[M+H] + .
Embodiment 3
[0099] The preparation of formula IV compound:
[0100] Add 227g (1mol) of the compound of formula III to a 2L three-neck flask, add 224g (4mol) of 85% potassium hydroxide and 900mL of water, heat to 98-100°C, keep stirring for 3-4 hours, and monitor the completion of the reaction by HPLC. Cool to 10°C, add 36% hydrochloric acid dropwise to about pH=2, a large amount of solids precipitate out, collect the solids by filtration, and air-dry at 50°C to obtain 181 g of a yellow compound of formula IV with a molar yield of 85% and a purity of 96%. 1 H-NMR (CDCl 3 , 400MHz) δ3.80(3H, s), 6.89(1H, s), 7.34(1H, s); MS(ESI, m / z): 214[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com