Synthetic method of (-)-Corey lactone diol
A technology of coreylactone diol and a synthetic method, which is applied in the synthetic field of coreylactonediol (Coreylactonediol), can solve problems such as inability to carry out large-scale production, instability of dichloride compounds, high price of alkaloids, etc. Achieve the effects of high resolution efficiency, short reaction time, and avoiding isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
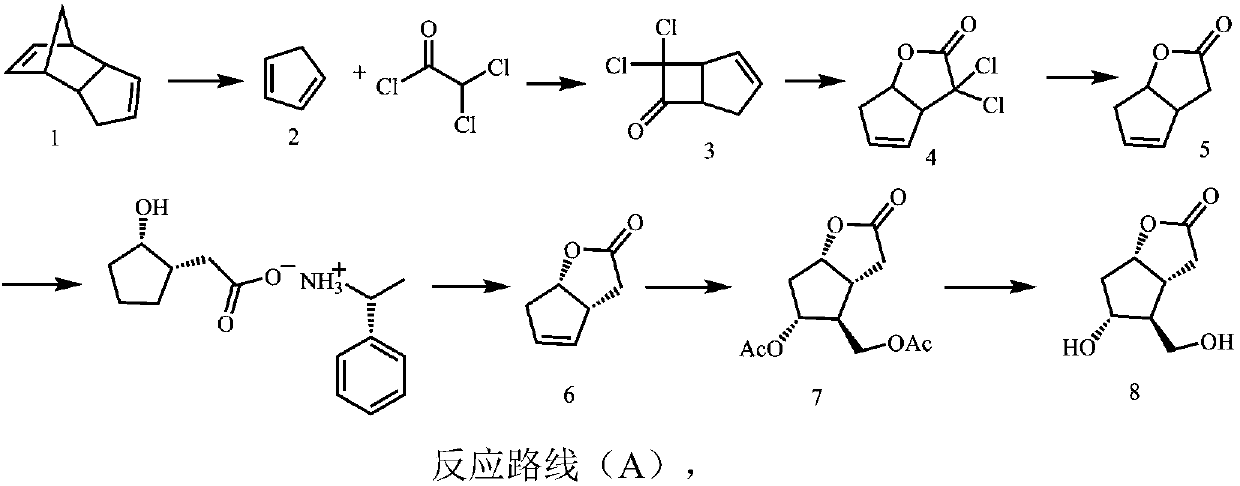
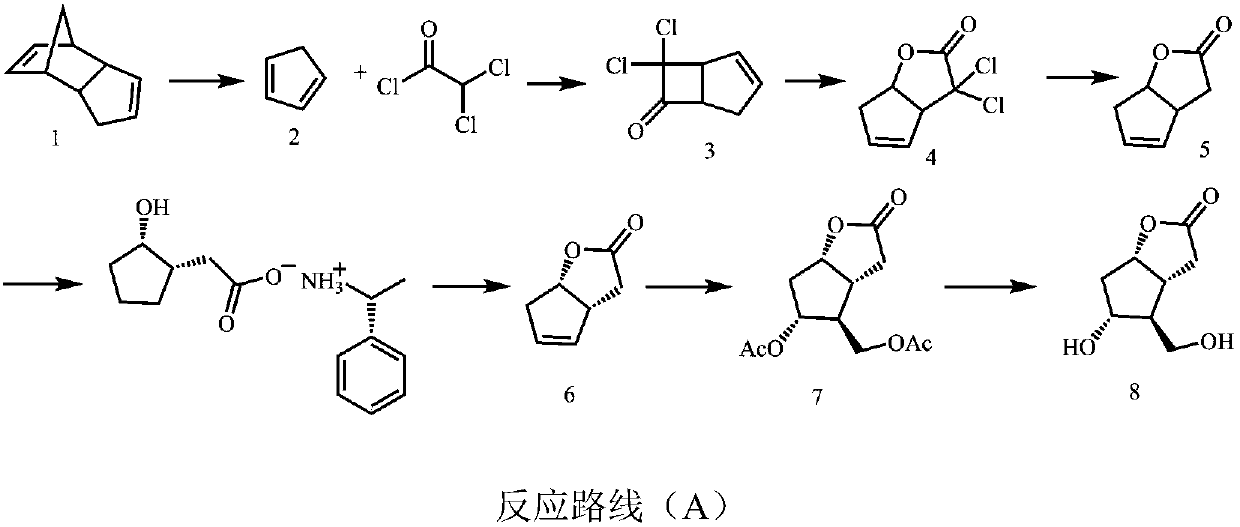
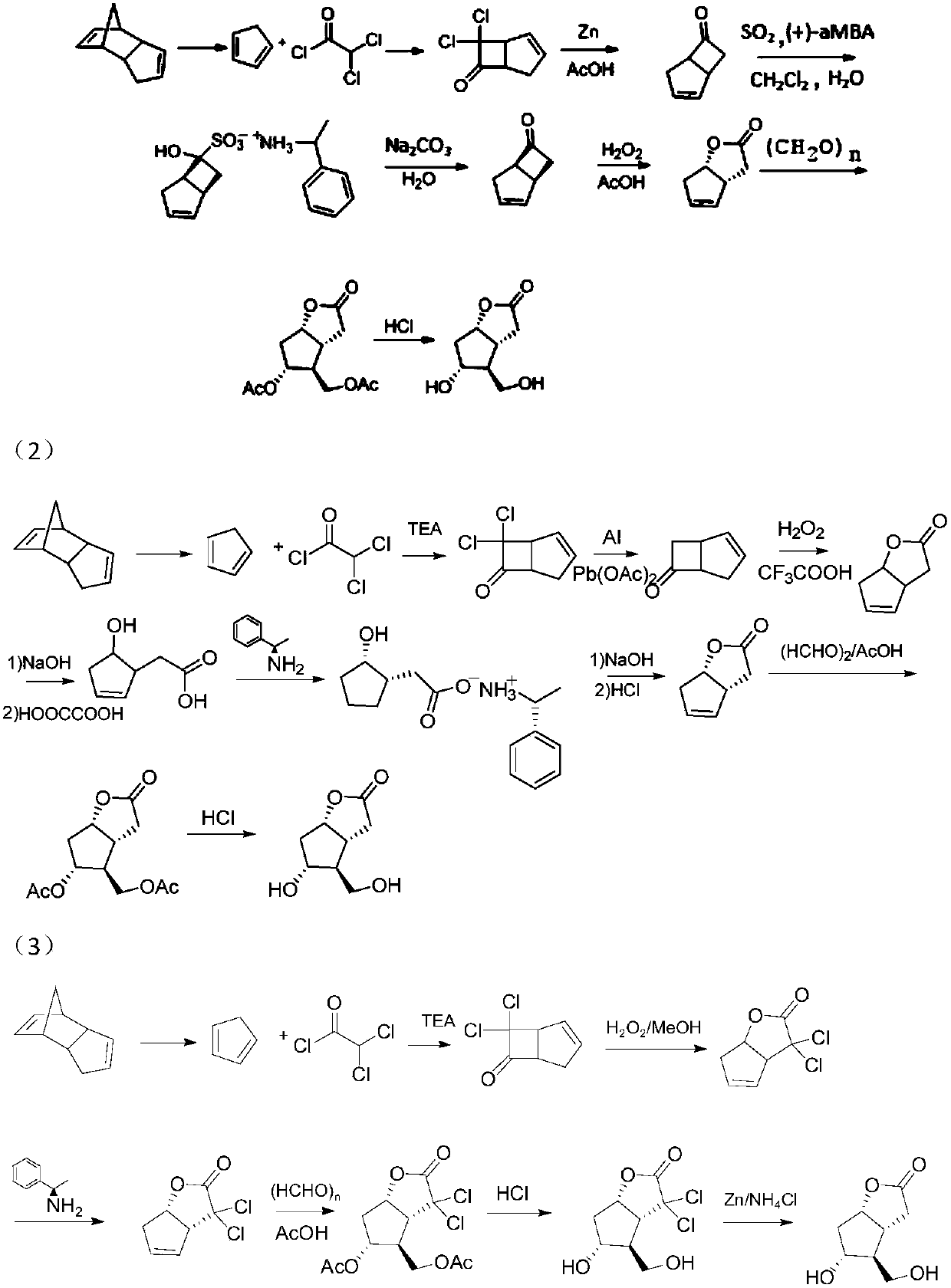
Image
Examples
Embodiment 1
[0065] The synthesis of embodiment 1, compound 2
[0066] Add 200g of dicyclopentadiene into a 500ml eggplant-shaped bottle, heat it under stirring until it is completely dissolved and clarified, carry out rectification with a rectification column, and collect fractions at 38-42°C to obtain 192g of compound 2 (1,3-cyclopentadiene ene), yield 96%.
Embodiment 2
[0067] The synthesis of embodiment 2, compound 3
[0068] Add 100g of dichloroacetyl chloride, 103g of compound 2 (1,3-cyclopentadiene) and 0.68L of n-heptane into a 2L four-necked flask, and slowly drop triethylamine (72.4g) into n-hexane Alkane solution, dropwise, stirred overnight at room temperature. The filter residue was suction filtered, the filtrate was washed with saturated brine, dried over anhydrous sodium sulfate, the solvent was removed by distillation under reduced pressure at low temperature, the residue was distilled under reduced pressure, and fractions at 50-54°C were collected to obtain 104 g of compound 3 with a yield of 86%.
[0069] 1 H NMR(500MHz,CHLOROFORM-d)ppm2.58(m,1H,CH),2.82(m,1H,CH),4.07(m,1H,CH),4.27(m,1H,CH),6.05(m ,1H,CH).
Embodiment 3
[0070] The synthesis of embodiment 3, compound 4
[0071] Add 100g of compound 3, 150ml of methanol, and 150ml of water into a 1L three-necked flask, place it in an ice-salt bath under stirring, and slowly add 110ml of 30% hydrogen peroxide dropwise at a controlled internal temperature of -5°C. After the drop is complete, slowly add 5N hydroxide Sodium aqueous solution, control the internal temperature not higher than 30°C, after dropping, stir overnight at room temperature.
[0072] Add sodium sulfite to quench excess hydrogen peroxide, after full reaction, add hydrochloric acid to adjust the acidity, filter out the salt in the system, extract with ethyl acetate, wash the organic phase with saturated brine, dry with anhydrous sodium sulfate, distill off the solvent under reduced pressure, and the residue Dissolve in methanol for clarification, then add n-pentane for crystallization, wash thoroughly, and filter out the solid with suction to obtain 479 g of white compound 4 wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com