Non-noble metal diatomic electrocatalyst, and preparation method and application thereof
An electrocatalyst and non-precious metal technology, which is applied in the field of non-noble metal diatomic electrocatalyst and its preparation, can solve the problems of increased surface free energy and poor stability, and achieve the effects of high current density, stable performance and small Tafel slope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
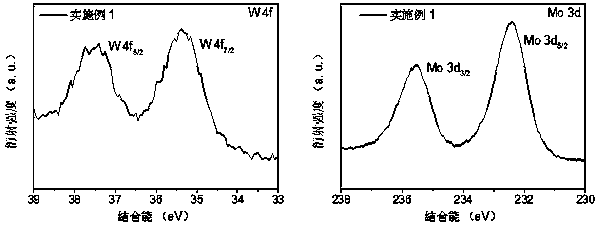
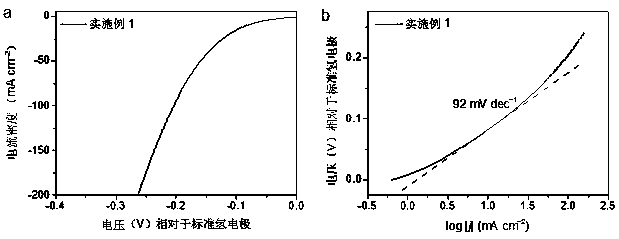
[0041] When the molar ratio of metal tungsten and molybdenum is 3:17. Accurately pipette 4.736 mL of dispersion A and accurately weigh 0.2368 g (NH 4 ) 6 Mo 7 o 24 • 4H 2 O was placed in the dispersion B for ultrasonic dispersion for 4 h, the pH of the solution was adjusted to >6.0, and then the mixture was heated to 190 oC Last for 12 hours. After the reaction was completed, the hydrothermal kettle was cooled, and the product was taken out, and the columnar product was frozen in liquid nitrogen, and then dried for 30 h with a freeze dryer to obtain an airgel. Set the furnace temperature to 800 o C, the gas flow rate is Ar: 150 sccm, NH 3 : 100 sccm, the total gas pressure is 2.85 Torr, after 3 h of reaction, a nitrogen-doped graphene composite anchored by W / Mo diatoms is obtained, W 3 Mo 17 -NG-3h.
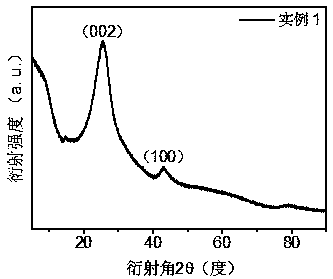
[0042] Such as figure 1 Shown, is the (002) and (100) diffraction peaks of the XRD pattern of this material, shows that W 3 Mo 17 -The existence of graphene and the...
Embodiment 2
[0046] When the molar ratio of metal tungsten and molybdenum is 7:13. Accurately pipette 15.98 mL of dispersion A and accurately weigh 0.4281 g (NH 4 ) 6 Mo 7 o 24 • 4H 2 O was placed in the dispersion B for ultrasonic dispersion for 4 h, the pH of the solution was adjusted to >6.0, and then the mixture was heated to 190 oC Last for 12 hours. After the reaction was completed, the hydrothermal kettle was cooled, and the product was taken out, and the columnar product was frozen in liquid nitrogen, and then dried for 30 h with a freeze dryer to obtain an airgel. Set the furnace temperature to 800 o C, the gas flow rate is Ar: 150 sccm, NH 3 : 100 sccm, the total gas pressure is 2.85 Torr, after 3 h of reaction, a nitrogen-doped graphene composite anchored by W / Mo diatoms is obtained, W 7 Mo 13 -NG-3h.
[0047] Such as Figure 4 Shown is the SEM image of the material, which only shows the fluffy wrinkled structure of graphene, and no nanoparticles appear, implying the...
Embodiment 3
[0054] When the molar ratio of metal tungsten and molybdenum is 7:13. Accurately pipette 15.98 mL of dispersion A and accurately weigh 0.4281 g (NH 4 ) 6 Mo 7 o 24 • 4H 2 O was placed in the dispersion B for ultrasonic dispersion for 4 h, the pH of the solution was adjusted to >6.0, and then the mixture was heated to 190 oC Last for 12 hours. After the reaction was completed, the hydrothermal kettle was cooled, and the product was taken out, and the columnar product was frozen in liquid nitrogen, and then dried for 30 h with a freeze dryer to obtain an airgel. Set the furnace temperature to 800 o C, the gas flow rate is Ar: 150 sccm, NH 3 : 100 sccm, the total gas pressure is 2.85 Torr, after 5 h of reaction, a nitrogen-doped graphene composite anchored by W / Mo diatoms is obtained, W 7 Mo 13 -NG-5h.
[0055] Such as Figure 10 Shown, is the (002) and (100) diffraction peaks of the XRD pattern of this material, shows that W 7 Mo 13-The existence of graphene and me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com