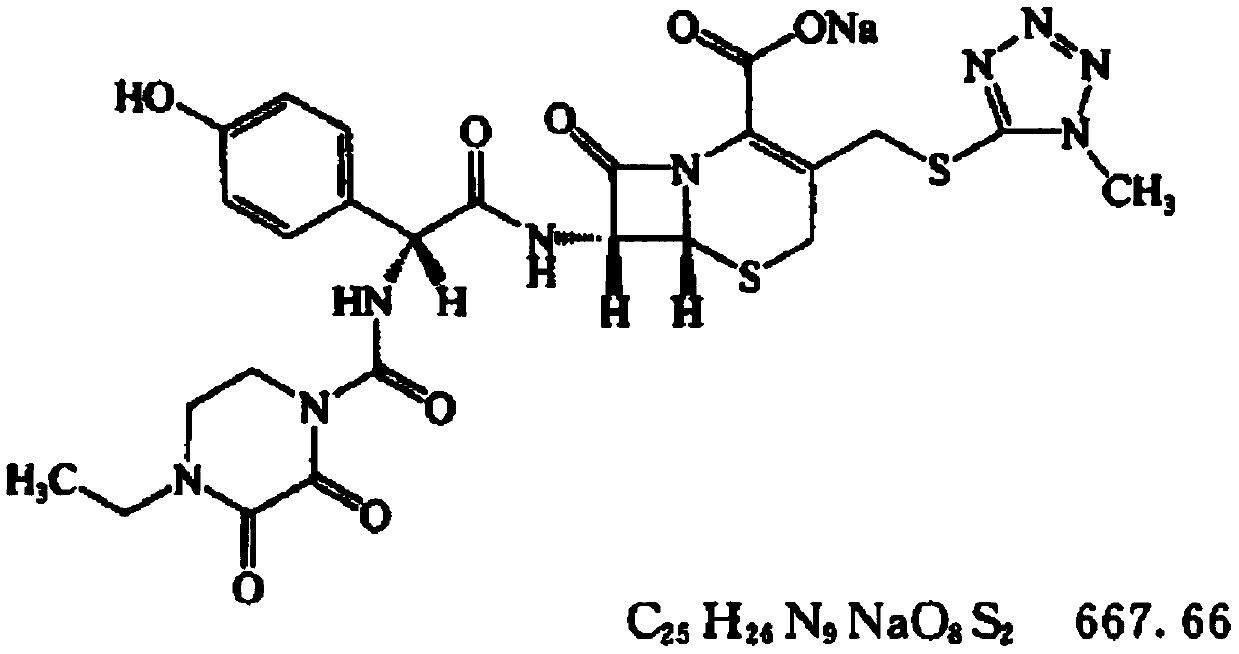
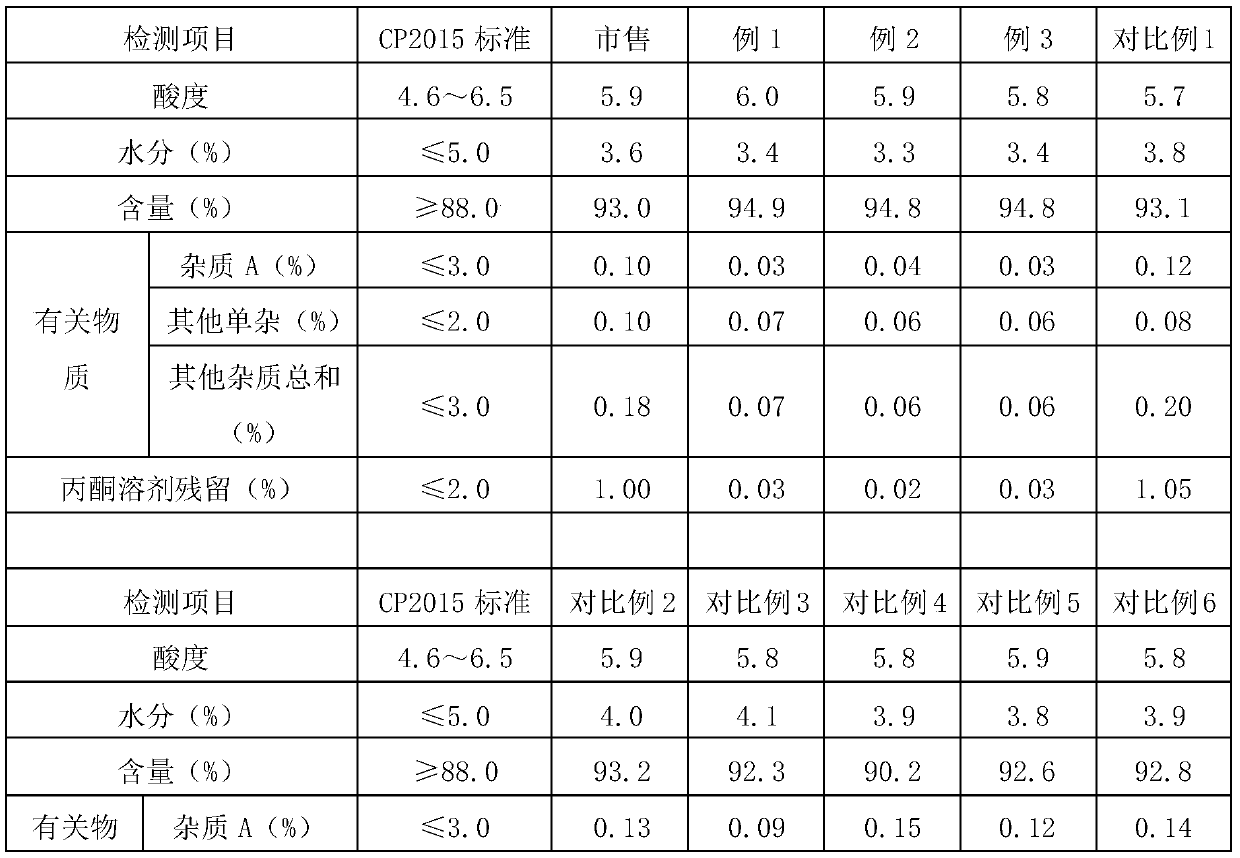
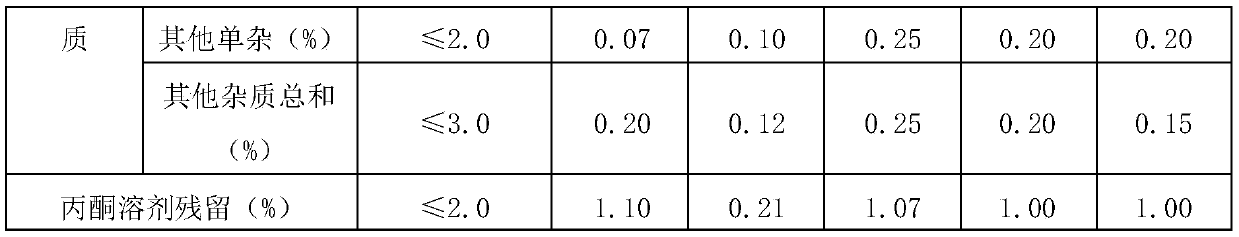
Preparation method of high-purity cefoperazone sodium micro powder
A technology of cefoperazone sodium micropowder and cefoperazone sodium, applied in the field of medicine, can solve problems such as solvent residues that cannot be effectively solved, and achieve the effects of high product yield and purity, few by-products, and avoiding oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of high-purity cefoperazone sodium micropowder, comprising: using cefoperazone acid as a raw material, first preparing a cefoperazone sodium solution, and then performing decolorization treatment, adding the decolorized cefoperazone sodium solution into a reaction kettle fed with a supercritical fluid, and crystallizing to obtain Cefoperazone Sodium Micropowder.
[0030] Concrete preparation method comprises the following steps:
[0031] A. Add cefoperazone acid to an organic solvent, add an alkaline sodium-containing reagent to adjust the pH to 6.5-7.0, and obtain a cefoperazone sodium solution; wherein the organic solvent is any one of acetone, ethanol, and isopropanol, and the alkaline sodium-containing The reagent is any aqueous solution of sodium bicarbonate, sodium carbonate or sodium hydroxide.
[0032] B. add decolorizing agent in the cefoperazone sodium solution and carry out decolouring, obtain filtrate and filter cake after filtering, wa...
Embodiment 1
[0039] A preparation method of high-purity cefoperazone sodium micropowder, comprising: using cefoperazone acid as a raw material, first preparing a cefoperazone sodium solution, and then performing decolorization treatment, adding the decolorized cefoperazone sodium solution into a reaction kettle fed with a supercritical fluid, and crystallizing to obtain Cefoperazone Sodium Micropowder.
[0040] Concrete preparation method comprises the following steps:
[0041] A. 10g cefoperazone acid is added in 100L acetone, and aqueous sodium bicarbonate solution is adjusted pH to 6.5, obtains cefoperazone sodium solution;
[0042] B. Decolorize the gac that accounts for 10% of the quality of the cefoperazone sodium solution in the cefoperazone sodium solution. The decolorization time is 30 to 60 minutes. After filtering, the filtrate and filter cake are obtained, and then the filter cake is washed, and the eluate and the filtrate are combined to obtain the decolorized cefoperazone sod...
Embodiment 2
[0048] A preparation method of high-purity cefoperazone sodium micropowder, comprising: using cefoperazone acid as a raw material, first preparing a cefoperazone sodium solution, and then performing decolorization treatment, adding the decolorized cefoperazone sodium solution into a reaction kettle fed with a supercritical fluid, and crystallizing to obtain Cefoperazone Sodium Micropowder.
[0049] Concrete preparation method comprises the following steps:
[0050] A. 30g cefoperazone acid is added in 100L ethanol, and sodium carbonate aqueous solution is adjusted pH to 7.0, obtains cefoperazone sodium solution;
[0051] B. decolorize the gac that accounts for 1% of the quality of the cefoperazone sodium solution in the cefoperazone sodium solution, the decolorization time is 30~60min, obtain the filtrate and filter cake after filtration, then wash the filter cake, obtain the decolorized cefoperazone sodium solution after the eluate and the filtrate are combined , the concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com