Electroluminescent material, preparation method and applications thereof
An electroluminescent material and luminescent technology, applied in the direction of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of not getting the expected color coordinates, weak electron-absorbing ability, strong electron-absorbing ability, etc., and reach the active site More points, stable molecular structure, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 ethyl 2-bromobenzoate
[0046] In a 500mL three-necked flask, 2-bromobenzoic acid (20.1g, 0.1mol) was dissolved in 200ml of ethanol, and 20ml of concentrated sulfuric acid was added dropwise to the reaction solution, and after stirring at room temperature for 12 hours, the reaction was stopped, and the reaction was quenched with water , extracted with dichloromethane and dried with anhydrous magnesium sulfate, the solution was concentrated to obtain a yellow liquid, which was purified by silica gel column chromatography, and the mixed solvent of petroleum ether / dichloromethane (7 / 1, v / v) was eluting agent, yield 84%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0047]
Embodiment 2
[0048] Example 2 Preparation of ethyl 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) ethyl benzoate
[0049] Under the protection of an inert gas, ethyl 2-bromobenzoate (17.5g, 76.4mmol) was dissolved in 250ml of anhydrous tetrahydrofuran, and a concentration of 2.4mol / L of n-butyllithium in n-hexane (38.2ml , 91.7mmol), after stirring at room temperature for 1 hour, 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborane (25.6g , 0.14mol), stop the reaction, quench the reaction with water, extract with dichloromethane and dry with anhydrous magnesium sulfate, get a khaki liquid after the solution is concentrated, purify by silica gel column chromatography, the mixing of petroleum ether / dichloromethane The solvent (5 / 1, v / v) was used as eluent, and a white solid was obtained with a yield of 87%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
...
Embodiment 3
[0051] Example 3 Preparation of diethyl 2,2'-(5,5-dioxodibenzo[b,d]phenyl-3,7-yl)bis(benzene-3-carboxylate)
[0052] Under an argon atmosphere, add ethyl 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethyl benzoate to a 500mL three-necked flask (8.4g, 30.4mmol), 2,7-dibromo-S,S-dioxodibenzophenone (20.0g, 45.6mmol), tetrabutylammonium bromide (0.49g, 1.52mmol), catalyst triphenyl Phosphine palladium dichloride (1.76g, 1.52mmol) and 200mL toluene were stirred and heated, and when the temperature was stabilized at 110°C, an organic base (20mL) and K 2 CO 3 (41.95g, 0.30mol) aqueous solution 42mL, reacted for 12h. After the reaction solution was concentrated, it was purified by silica gel column chromatography, using a mixed solvent of petroleum ether and dichloromethane (3 / 1, v / v) as eluent, to obtain a light yellow solid with a yield of 75%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
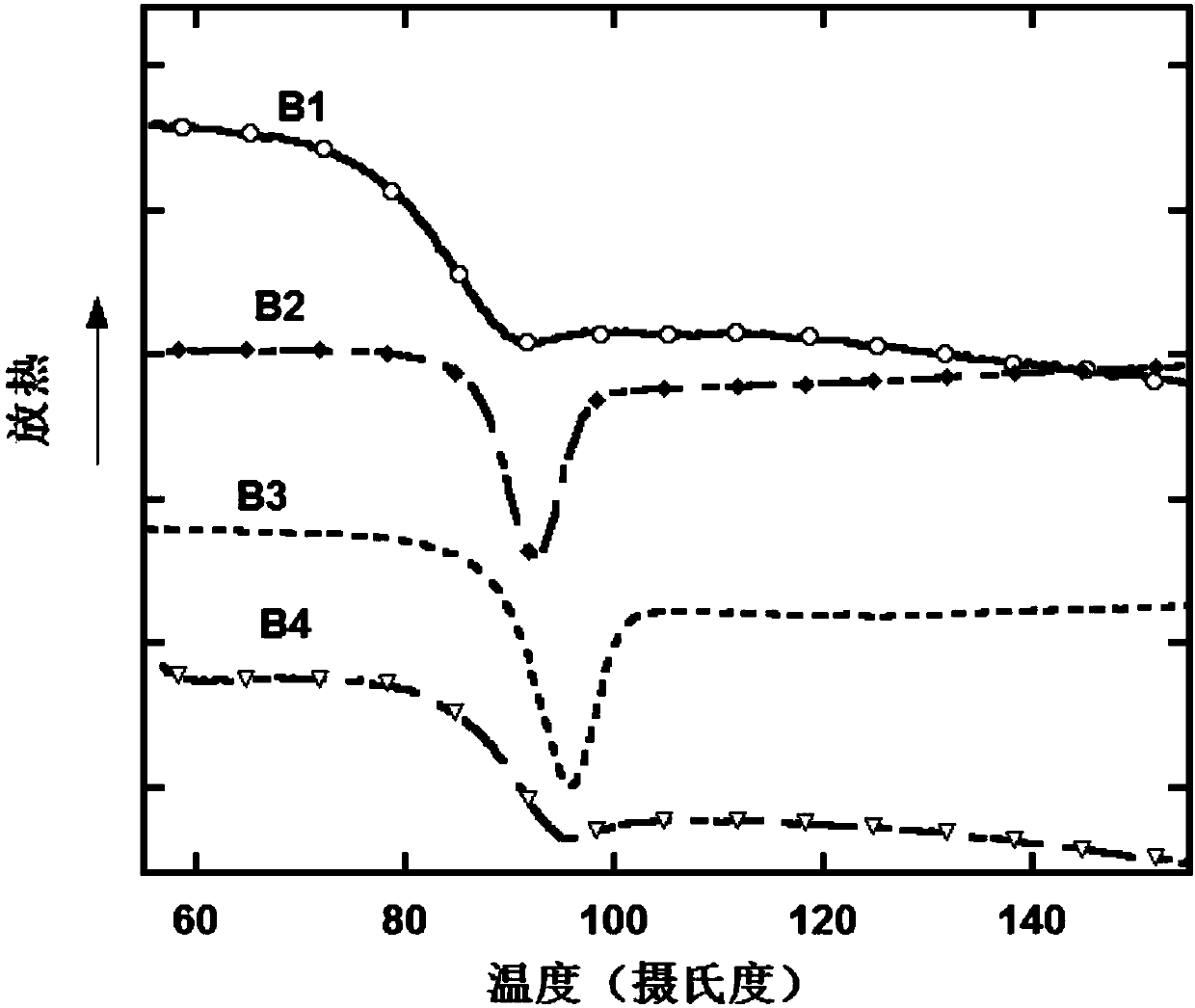
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com