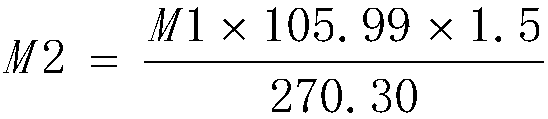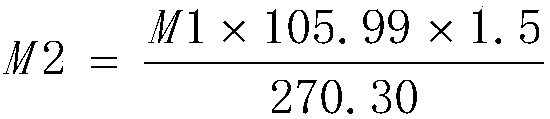Preparation method of iron sucrose composite solution with low heavy metal content, and product
A technology with low heavy metals and complexes, applied in the field of medicine, can solve the problems of low removal rate of heavy metals, difficulties in development and stable production, etc., and achieve the effects of no accumulation risk of heavy metals, high safety, and low content of heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 110g of anhydrous sodium carbonate and 2.3kg of purified water into the 5L glass flask, stir for about 30min to dissolve, heat to 80°C, then add 250g of ferric chloride hexahydrate (manganese content 934ppm, nickel content 48.12ppm, cobalt content 9.88ppm) ), stirred and reacted for about 15min, the solution was black, filtered, and the filtrate was transferred to a 5L glass flask and cooled to 5°C. Add 20% sodium carbonate solution dropwise for about 5h to adjust the pH of the reaction solution to about 2.0, stir the reaction at 5°C for about 4h, adjust the pH of the reaction solution to about 7.0 with 20% sodium carbonate solution, stir for 30min and filter, the filter cake uses about 5kg The purified water was repeatedly washed 4 times, the conductivity of the washed purified water was 3342 μs / cm, the obtained ferric hydroxide colloid was added with sodium hydroxide to adjust the pH of the reaction solution to not less than 10, 1.3 kg of sucrose was added, and the...
Embodiment 2
[0047] Add 110g anhydrous sodium carbonate and 2.3kg purified water to the 5L glass flask, stir for about 30min to dissolve, heat to 60°C, then add 250g ferric chloride hexahydrate (manganese content 934ppm, nickel content 48.12ppm, cobalt content 9.88ppm) ), stirred and reacted for about 15min, the solution was black, filtered, and the filtrate was transferred to a 5L glass flask and cooled to 0°C. Add 20% sodium carbonate solution dropwise for about 5h to adjust the pH of the reaction solution to about 3.0, stir the reaction at 5°C for about 8h, adjust the pH of the reaction solution to about 8.5 with 20% sodium carbonate solution, stir for 30min and filter, the filter cake uses about 5kg The purified water was repeatedly washed 4 times, the conductivity of the washed purified water was 2490 μs / cm, the obtained ferric hydroxide colloid was added with sodium hydroxide to adjust the pH of the reaction solution to not less than 10, 1.0 kg of sucrose was added, and the reaction s...
Embodiment 3
[0049] Add 105g of anhydrous sodium carbonate and 2.3kg of purified water to the 5L glass flask, stir for about 30min to dissolve, heat to 50°C, then add 250g of ferric chloride hexahydrate (manganese content 934ppm, nickel content 48.12ppm, cobalt content 9.88ppm) ), stirred and reacted for about 15min, the solution was black, filtered, and the filtrate was transferred to a 5L glass flask and cooled to 5°C. Add 20% sodium carbonate solution dropwise for about 3h to adjust the pH of the reaction solution to about 2.3, stir the reaction at 5°C for about 8h, adjust the pH of the reaction solution to about 8.2 with 20% sodium carbonate solution, stir for 30min and filter, the filter cake uses about 5kg The purified water was repeatedly washed 5 times, and the conductivity of the washed purified water was 1216 μs / cm. The obtained ferric hydroxide colloid was added with sodium hydroxide to adjust the pH of the reaction solution to not less than 10, 0.9 kg of sucrose was added, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com