A kind of synthetic method of 3-hydroxy desloratadine metabolite
A technology of loratadine and synthetic method, which is applied in the field of synthesis of 3-hydroxydesloratadine metabolites, can solve the problem of unsuitable 3-hydroxydesloratadine metabolites, low product yield, and expensive raw materials and other issues, to achieve the effect of great application research value, reasonable process design, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
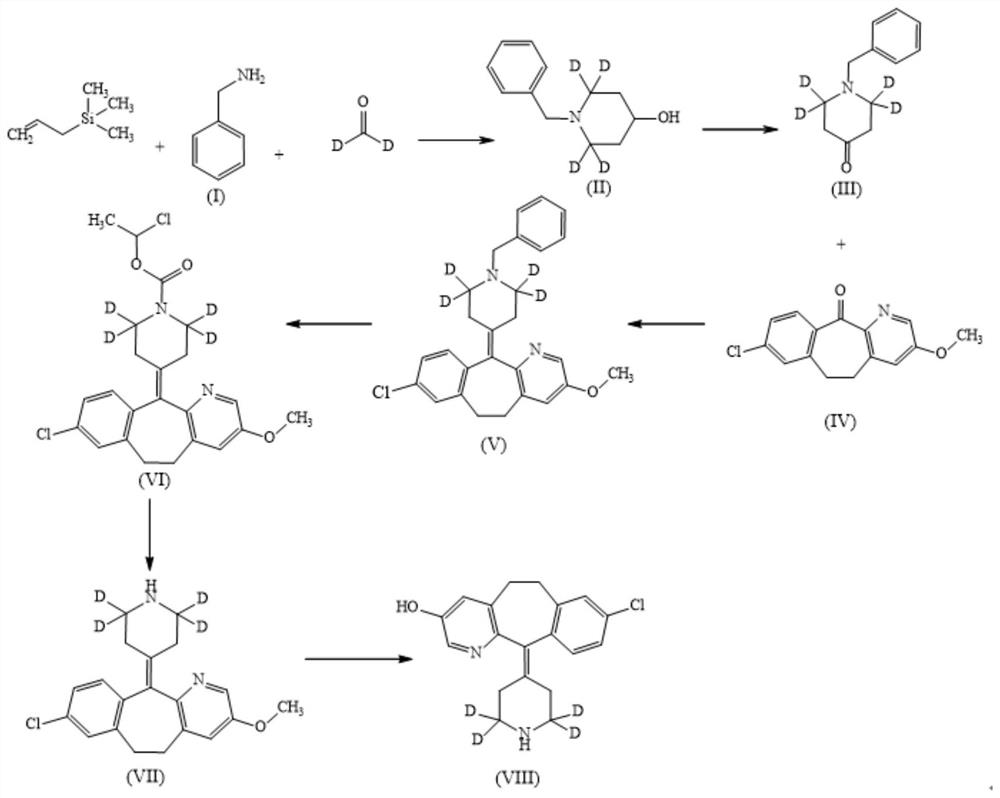
[0022] Such as figure 1 Shown, a kind of synthetic method of 3-hydroxy desloratadine metabolite comprises the following steps:
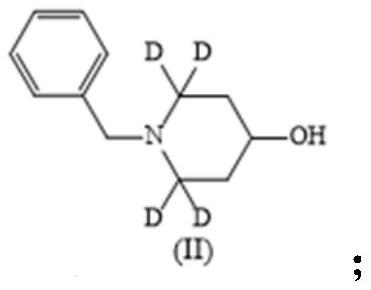
[0023] (1) Take 20 g of benzylamine hydrochloride I, put it into a 150 mL round bottom flask, add 6 g of 20 % deuterium 2 labeled formaldehyde heavy aqueous solution, 16 mL of allyltrimethylsilane, at 40 °C React for 48 hours; use 1M sodium hydroxide solution to adjust the pH of the reaction solution to 11, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 12g of intermediate II as a yellow oil, with a yield of 66.3% ;
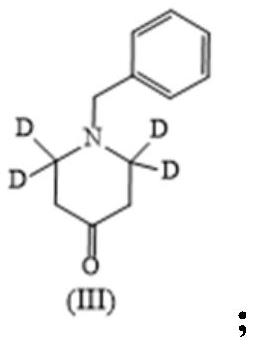
[0024] (2) Dissolve 6 g of intermediate II in 60 mL of dry toluene, add 14 g of Dess-Martin oxidant, react at 60°C for 8 hours, add 200 mL of water, extract with dichloromethane, and separate the dichloromethane phase Come out, concentrate, and purify by column chromatography, obtain 5g intermediate III, be white solid, yield is 84.2%;
[0025] (3) Suspend 15 g of al...
Embodiment 2
[0029] Such as figure 1 Shown, a kind of synthetic method of 3-hydroxy desloratadine metabolite comprises the following steps:
[0030] (1) Take 20 g of benzylamine trifluoroacetate I, put it into a 150 mL round bottom flask, add 6 g of 20% deuterium 2 labeled heavy formaldehyde solution, 14 mL of allyltrimethylsilane, 60 Reaction at ℃ for 48 hours; use 1M sodium hydroxide solution, adjust the pH of the reaction solution to 11, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 10 g of intermediate II as a yellow oil. rate 56.7%;
[0031] (2) Dissolve 6 g of intermediate II in 60 mL of dry toluene, add 12 g of PCC oxidant, react at 40 ° C for 24 hours, add 200 mL of water, extract with dichloromethane, and separate the dichloromethane phase, Concentrate and purify by column chromatography to obtain 4g of intermediate III as a white solid with a yield of 67.36%;
Embodiment 3
[0036] Such as figure 1 Shown, a kind of synthetic method of 3-hydroxy desloratadine metabolite comprises the following steps:
[0037] (1) Take 20 g of benzylamine hydrochloride Ⅰ, put it into a 150 mL round bottom flask, add 6 g of 20 % deuterium 2-labeled formaldehyde heavy aqueous solution, 16 mL of allyltrimethylsilane, at 60 °C React for 12 hours; use 1M sodium hydroxide solution to adjust the pH of the reaction solution to 11, extract with dichloromethane, concentrate the dichloromethane phase, and purify by column chromatography to obtain 11.3 g of intermediate II as a yellow oil, with a yield of 62.4 %;
[0038] (2) Dissolve 6 g of intermediate II in 60 mL of dry toluene, add 20 g of manganese dioxide oxidant, react at 60 °C for 8 hours, filter, add 200 mL of water, extract with dichloromethane, dichloromethane The phases were separated, concentrated, and purified by column chromatography to obtain 4.6 g of intermediate III as a white solid with a yield of 77.5%;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com