Fluorescent probe for detecting thiocyanate radicals as well as preparation method and application thereof
A fluorescent probe and thiocyanate technology, applied in the field of analytical chemistry, can solve the problems of low sensitivity and large effect, and achieve the effects of strong anti-interference ability, fast response speed and simple purification method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
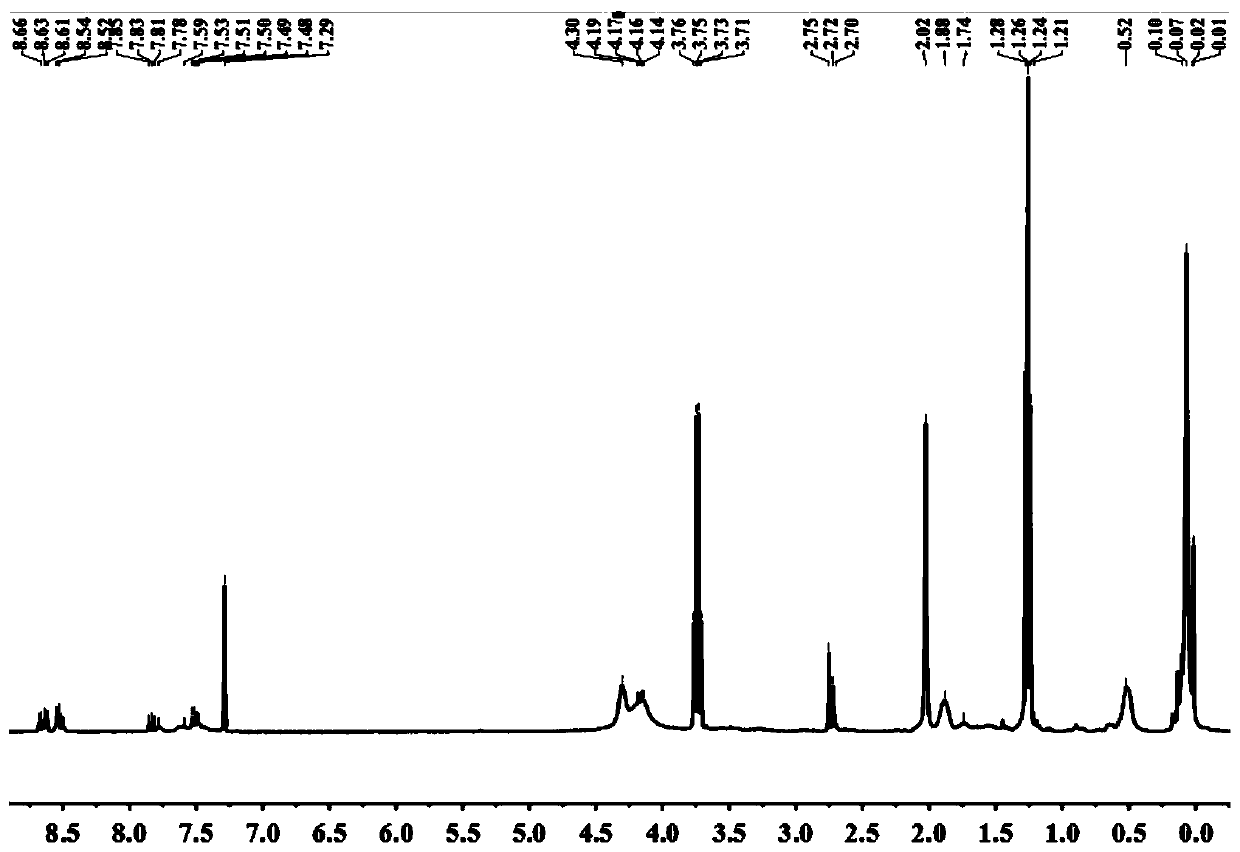
[0040] Example 1 Synthesis of Fluorescent Probes
[0041] (1) Dissolve 2.77 g of 4-bromo-1,8-naphthalene anhydride in 50 mL of ethanol, then dissolve 3 g of sodium methyl mercaptide in 50 mL of ethanol, and then add them together into a 250 mL eggplant-shaped reaction flask, at room temperature The reaction was stirred for 24 h. After the reaction, 100 mL of water was added to the reaction flask to obtain a yellow precipitate, which was filtered and dried to obtain a yellow solid, which was purified by column chromatography, and the eluent was methanol:dichloromethane (V / V)=1:20, to obtain Pure compound A:
[0042] ;
[0043] (2) Weigh 11.94 g of aminopropyl double-head and add 50 mL of absolute ethanol to dissolve, then weigh 4.8 g of glacial acetic acid, and slowly add it to the system; then weigh 6.38 g of glyoxal and 4.87 g of formaldehyde and mix well Add to the system; react at room temperature for 6h; add anhydrous MgSO 4 Remove the water in the system; after suct...
Embodiment 2
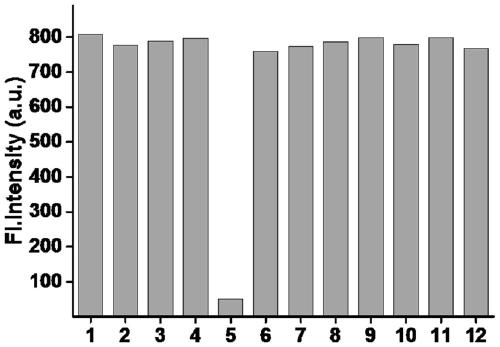
[0047] Example 2 Selectivity of fluorescent probes to different anions
[0048] Prepare 5 mL of various conventional anions in PBS aqueous solution (pH=7.4) with a concentration of 1 mM and the fluorescent probe mother solution obtained in Example 1 with a concentration of 1 mM as spares.
[0049] Add the probe mother solution and each anion solution respectively, the final concentration of the probe is 10 μM, the final concentration of the selective anion is 0.1 mM, and the volume is adjusted to 3 mL with phosphate buffer solution PBS, and the fluorescence detection (λ ex =405 nm, λ em =520nm), establish a histogram of fluorescence intensity and each ion, such as figure 2 As shown, 1-12 added sodium chloride, sodium acetate, sodium sulfide, sodium bicarbonate, sodium thiocyanate, sodium bisulfate, sodium nitrite, sodium nitrate, sodium sulfate, sodium carbonate, and probes. Depend on figure 2 It can be found that other ions have almost no effect on the fluorescence of th...
Embodiment 3
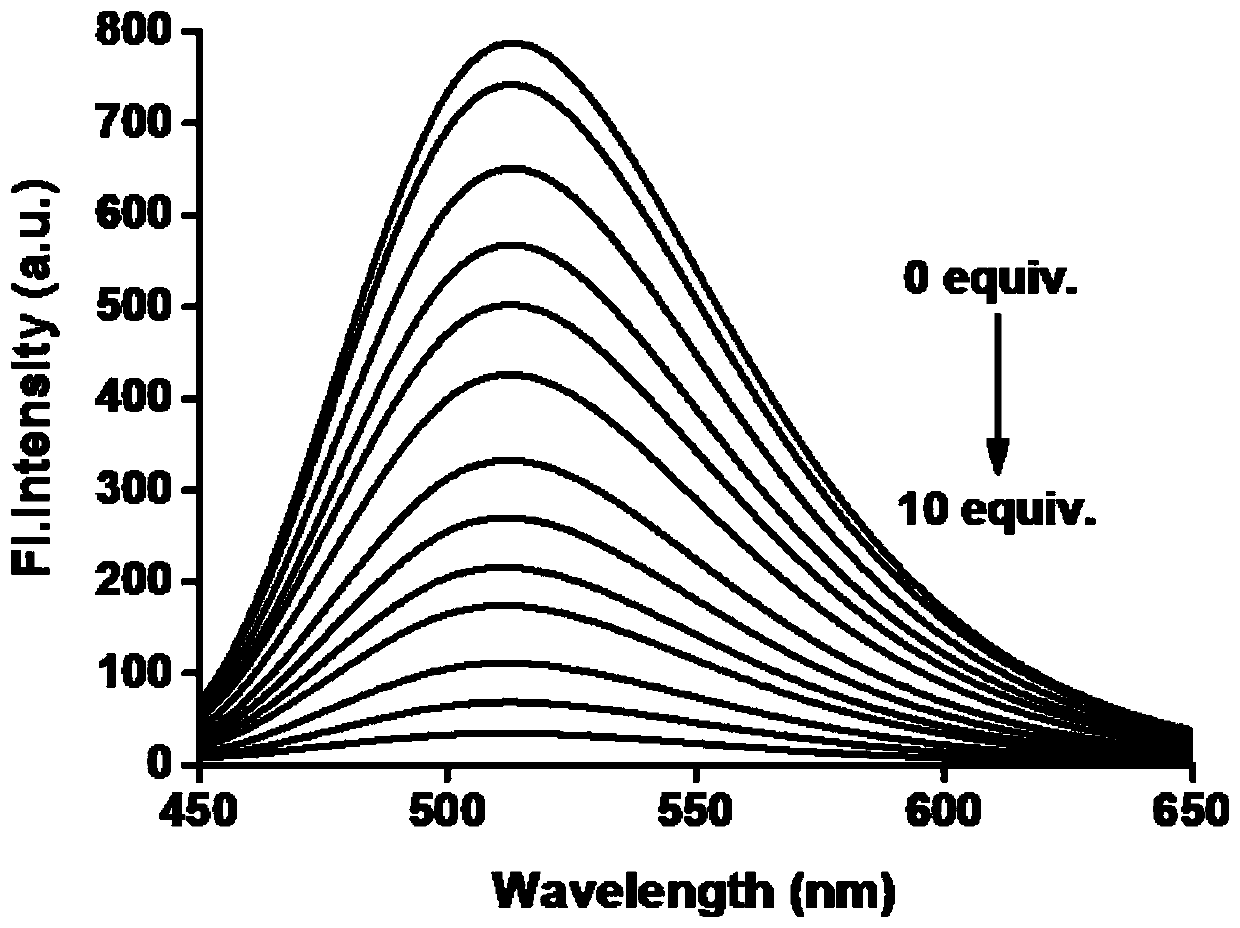
[0050] Example 3 Fluorescence titration detection of different concentrations of thiocyanate on the probe
[0051] Prepare 10 mL of an aqueous solution with a concentration of 1 mM thiocyanate and the fluorescent probe mother solution obtained in Example 1 with a concentration of 1 mM as backup.
[0052]The final concentration of the prepared probe was 10 μM, which interacted with different concentrations of thiocyanate (0, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100 μM), and carried out Fluorescence detection (λex=405 nm, λem=520 nm), calculate the fluorescence intensity in each system, and establish a standard curve of fluorescence intensity and thiocyanate concentration, such as image 3 . Depend on image 3 It can be seen that as the concentration of thiocyanate increases, the fluorescence intensity of the reaction system decreases gradually.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com