Boron-containing organic electroluminescent material, preparation method and application thereof
An electroluminescent material and organic technology, applied in the fields of luminescent materials, organic chemistry, silicon-organic compounds, etc., can solve the problems of low device life and efficiency, immature blue light-emitting devices, etc., and achieve the improvement of current efficiency and device life, Good prospects for industrialization and the effect of increasing the three-dimensional structure of space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
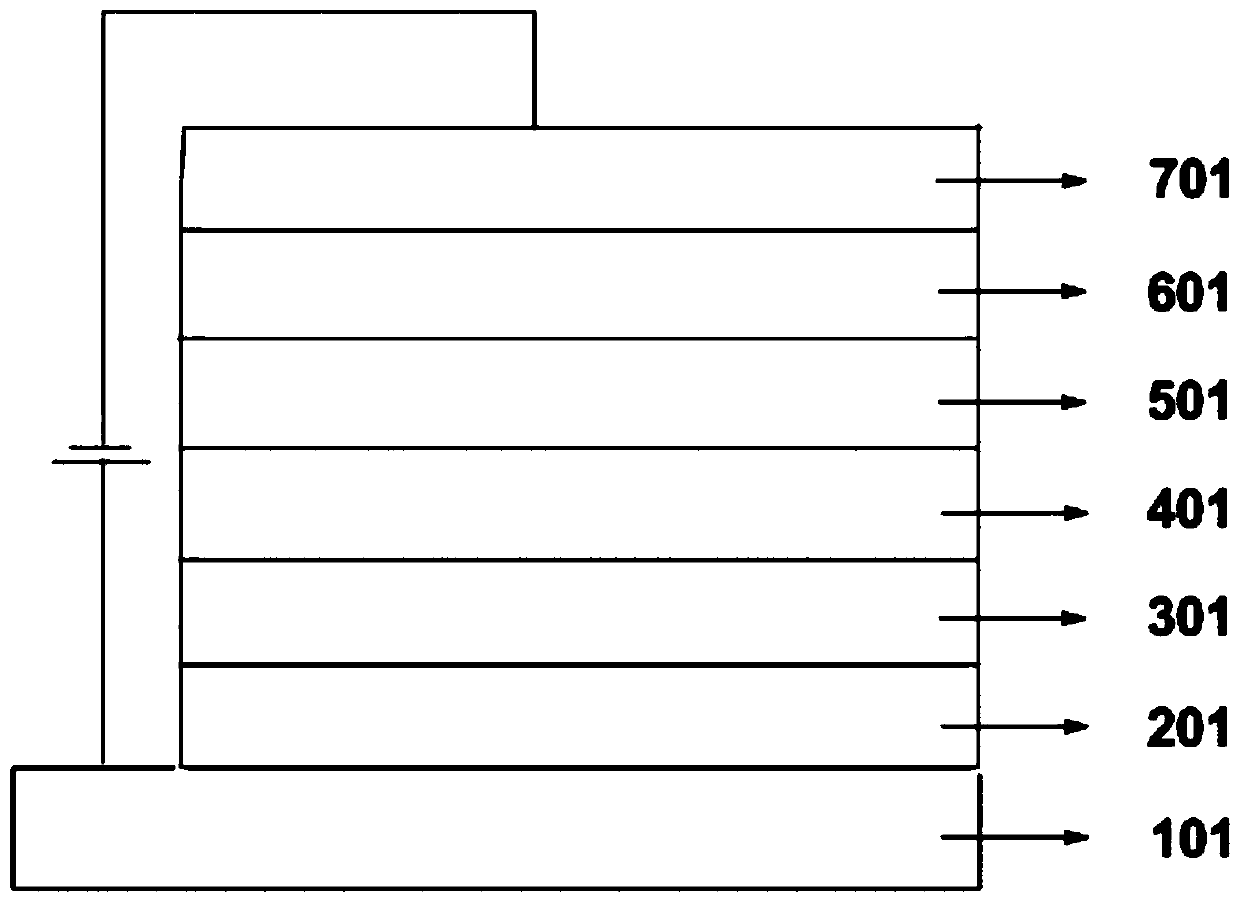
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 compound C01
[0052]
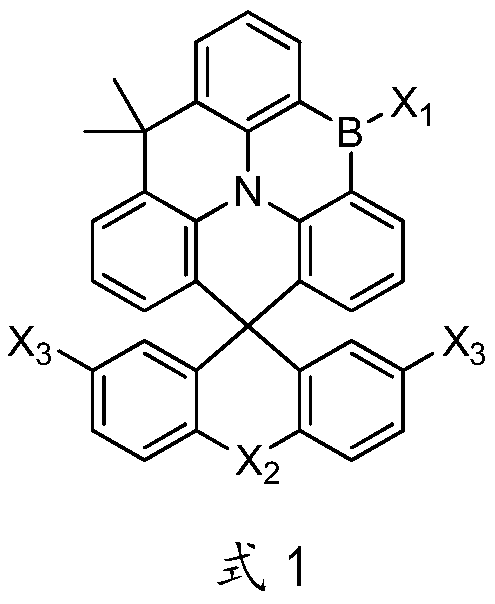
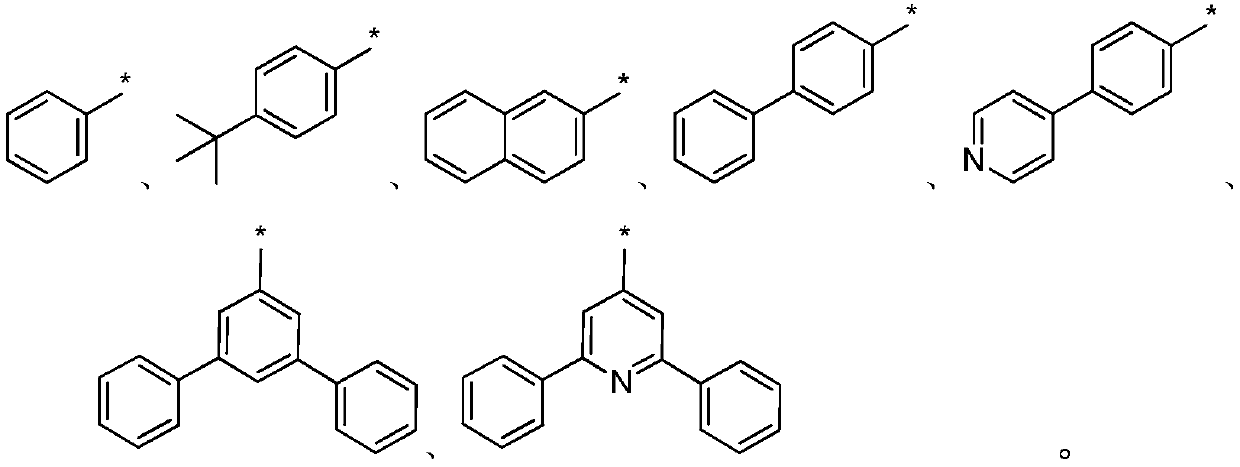
[0053]Preparation of intermediate C01-A: Dissolve 1-bromodiphenyl ether (2.49g, 0.01mol) in 100mL of dry tetrahydrofuran, under nitrogen protection, when the internal temperature of the system drops to -75°C, slowly add 4.8mL of Butyllithium n-hexane solution (2.5mol / L), after dropping, react at -75°C for 2 hours, after keeping warm, add 9,9-dimethyl-5H, 9H-quinoline [3,2,1-de ] acridone solid (2.80g, 0.009mol), add and heat preservation reaction for 2h, heat preservation is completed, continue heat preservation reaction at room temperature for 2h, then drop 70g of dilute hydrochloric acid with a mass concentration of 10%, stir for 1h, separate liquids, collect the organic phase, Remove the solvent to obtain 5 g of oil; then add 60 g of glacial acetic acid and 1.0 g of concentrated hydrochloric acid with a concentration of 36.5 wt % to the obtained oil, heat up to reflux, keep the temperature for 5 hours, cool d...
Embodiment 2
[0056] The preparation of embodiment 2 compound C04
[0057]
[0058] The preparation method of compound C04 is the same as the preparation of compound C01 in Example 1, and the difference is: adopt 4-bromobiphenyl (2.33g, 0.01mol) to replace bromobenzene (1.57g, 0.01mol) in Example 1, three The amount of boron bromide in methylene chloride was increased from 50 mL to 70 mL to obtain compound C04, 2.52 g of off-white solid powder, with a yield of 40.3%.
[0059] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 46 h 32 BNO, theoretical value 625.2577, test value 625.5514.
Embodiment 3
[0060] The preparation of embodiment 3 compound C07
[0061]
[0062] The preparation method of compound C07 is the same as the preparation of compound C01 in Example 1, the difference is that: 4-chloro-2,6-diphenylpyridine (2.66g, 0.01mol) is used to replace bromobenzene (1.57 g, 0.01mol), the amount of boron tribromide dichloromethane solution increased from 50mL to 80mL, to obtain compound C07, 3.16g off-white solid powder, yield 45.0%.
[0063] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 51 h 35 BN 2 O, theoretical value 702.2842, test value 702.4426.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com