Sulfur-bridged quinazoline tetradentate platinum complex phosphorescent material and preparation method thereof
A technology of quinazolines and platinum complexes, which is applied in the direction of luminescent materials, platinum-based organic compounds, platinum-group organic compounds, etc., can solve the problems of low quantum efficiency, improve luminous efficiency, improve color purity, and reduce interaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1,2-(3-bromophenyl)-4-diphenylaminoquinazoline qba
[0030] Weigh 0.35g (1mmol) of 2-(3-bromophenyl)-4-chloroquinazoline, 0.08g (2mmol) of 60% sodium hydride, and 0.25g (1.5mmol) of diphenylamine in a two-necked bottle, and add Solvent dimethyl sulfoxide, react at 30°C for 10h. After the reaction was completed, the reaction solution was poured into water, a large amount of white precipitate was produced, suction filtered, column chromatography, separated by petroleum ether:ethyl acetate=20:1 developer chromatography, and 0.3g (0.67mmol) of white solid qba was obtained , the yield was 67%. 1H NMR (400MHz, CDCl3) δ7.93 (m, 8H), 7.46 (dd, J = 5.3, 3.2Hz, 4H), 7.12 (dd, J = 5.4, 3.1Hz, 4H), 5.84 (S, 2H) .
[0031]
Embodiment 2
[0032] Embodiment 2, the quinazoline S (qba) of ligand sulfur atom bridging 2 preparation of
[0033] Weigh 2-(3-bromophenyl)-4-diphenylaminoquinazoline qba 0.225g (0.5mmol), potassium phosphate 0.064g (0.3mmol), potassium thioacetate 0.029g (0.25mmol), bis( Dibenzylideneacetone)palladium 0.023g (0.025mmol), 1,1-bis(diphenylphosphino)ferrocene 0.02g (0.035mmol), add toluene 3ml, acetone 1.5ml mixed solvent under nitrogen protection, 110℃ Reaction 7h. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, dried by adding anhydrous sodium sulfate, and separated by column chromatography with petroleum ether: ethyl acetate = 10:1 developer chromatography to obtain 0.1 g of white solid S(qba) 2 , the yield was 52%. 1H NMR (400MHz, CDCl3) δ = 8.38 (S, 2H), 8.11 (d, J = 7.6, 2H), 7.96 (d, J = 7.9, 2H), 7.65 (t, J = 7.7, 2H), 7.39 –7.28(m,17H),7.17(t,J=11.1,16H).
[0034]
Embodiment 3
[0035] Embodiment 3, complex S (qba) 2 Preparation of Pt
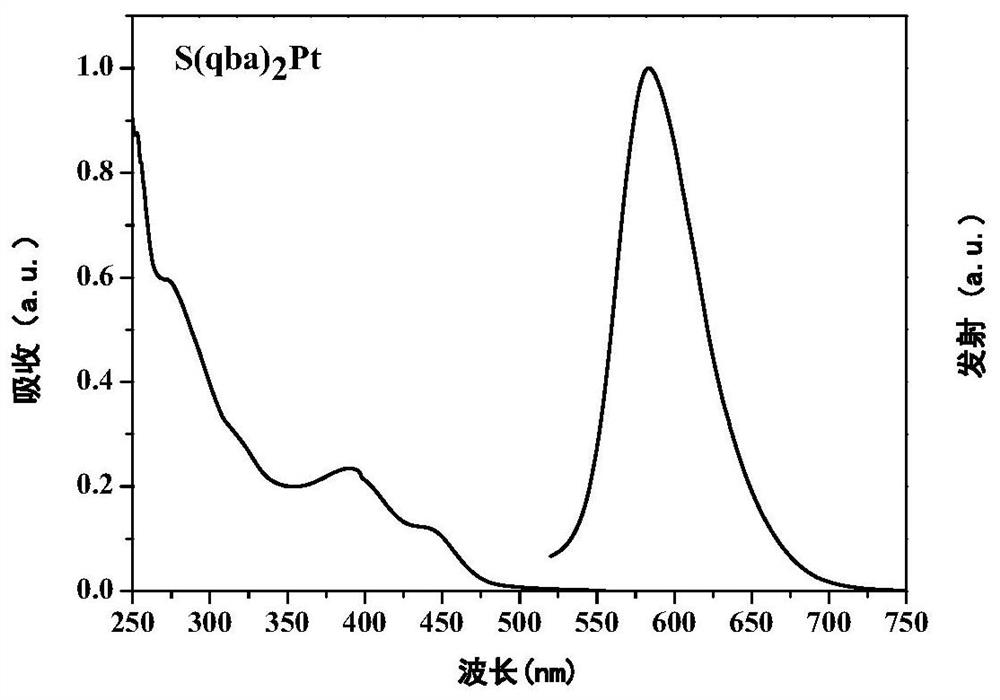
[0036] Weigh quinazoline S(qba) bridged by sulfur atom 2 0.077g (0.1mmol), 0.05g (0.1mmol) potassium tetrachloroplatinate, 4mg tetrabutylammonium bromide, 5ml acetic acid was added under nitrogen protection, stirred at room temperature for 12 hours, heated to 120°C for 70h. After the reaction was completed, cool to room temperature, add 15ml of deionized water, a precipitate precipitated out, filter with suction, dry, column chromatography, and use dichloromethane: ethyl acetate = 5:1 developer chromatography to obtain 0.032g orange-red solid S(qba) 2 Pt, 25% yield. 1H NMR (400MHz, CDCl3) δ=8.14–8.07(m,2H),7.47(t,J=7.7,4H),7.42–7.28(m,9H),7.09(t,J=7.6,1H),6.99 –6.93(m,2H).MALDI-TOF-MS(m / z):calcd for[M]+C 52 h 34 N 6 SPt: 969.37, found: 969.594.
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com