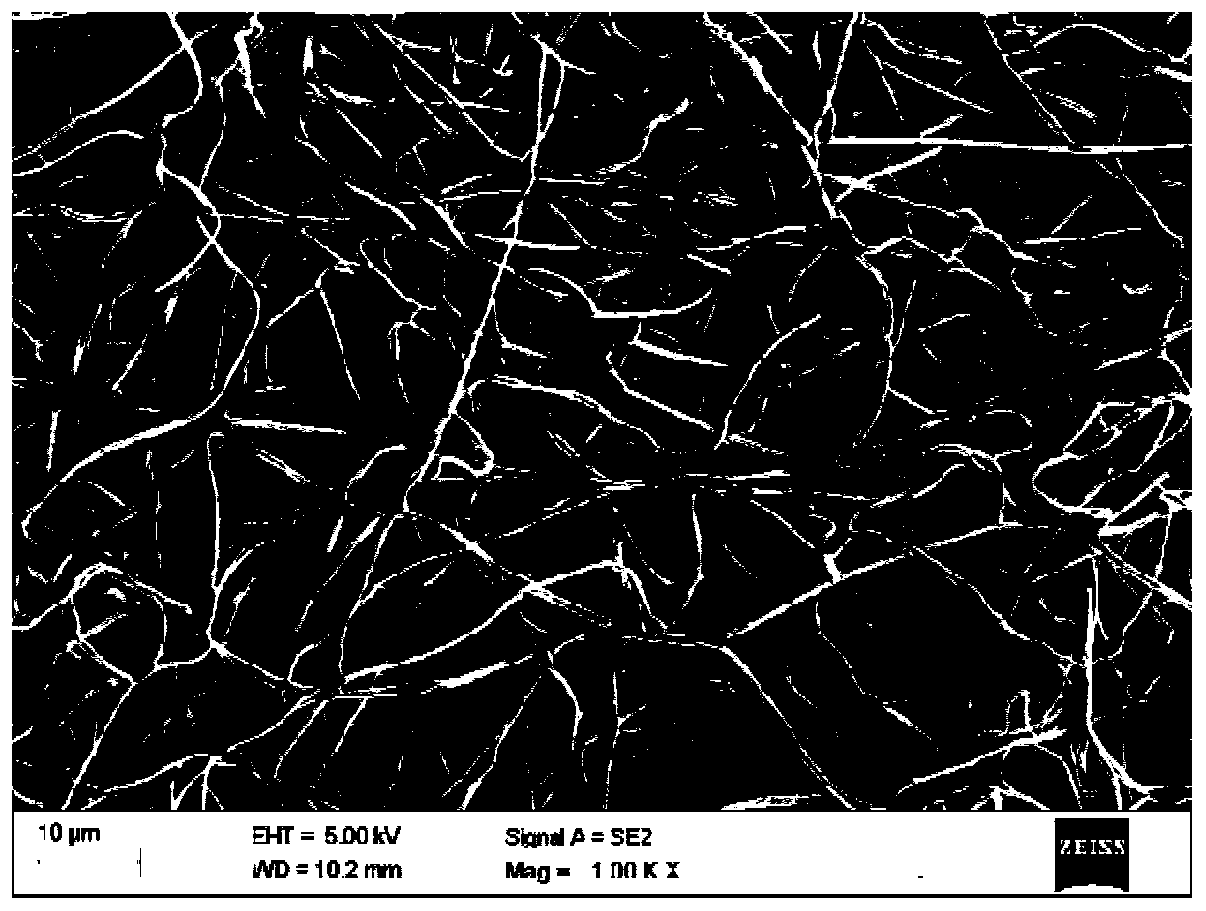
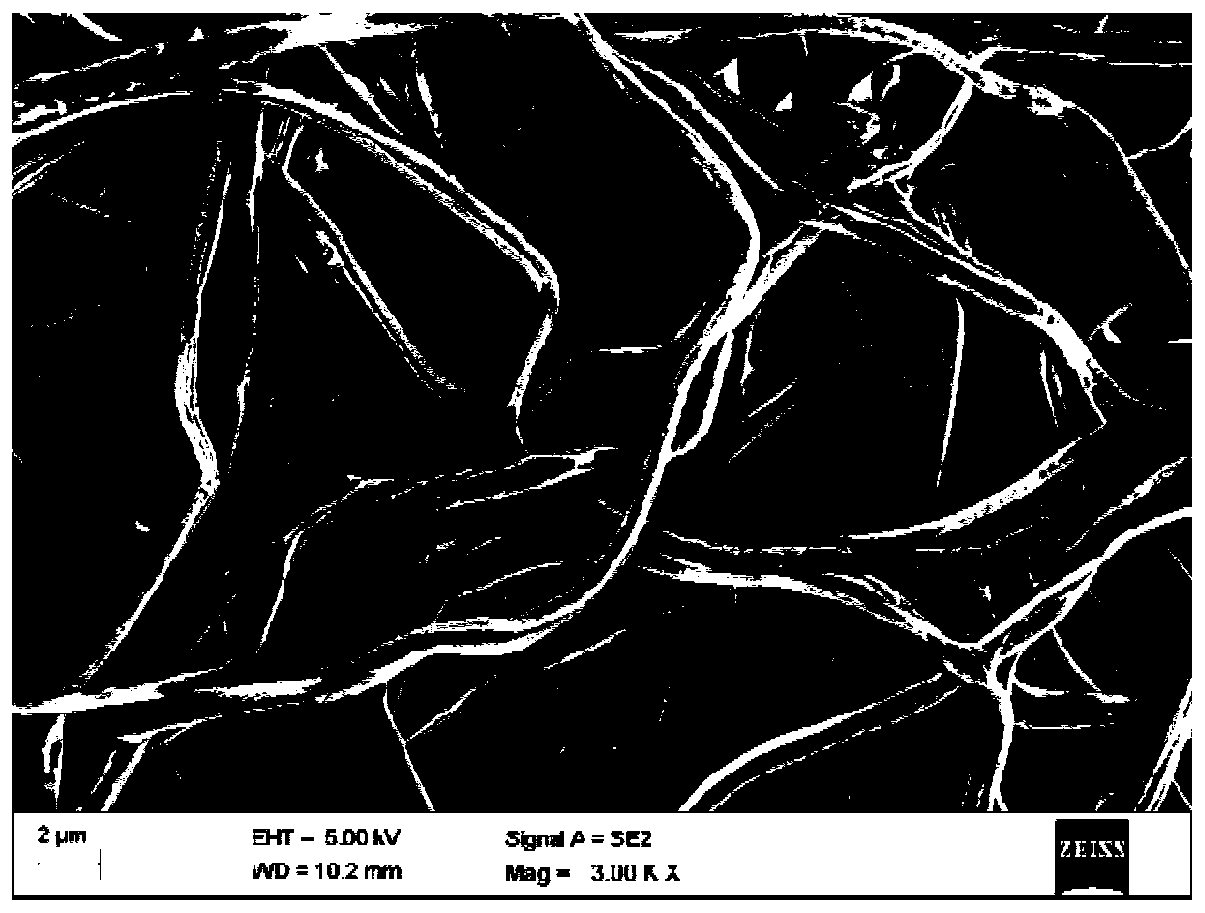
Preparation method of ibuprofen transdermal sustained-release preparation
A slow-release preparation and transdermal technology, which is applied in the fields of anti-inflammatory agents, pharmaceutical formulations, rayon manufacturing, etc., can solve problems such as difficult to inhibit the crystallization of drugs, and achieve the effects of good conductivity, crystallization inhibition, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of ibuprofen transdermal slow-release preparation, the steps are as follows:
[0040] (1) Polylactic acid (PLA, viscosity average molecular weight Mv is 10 0000) is dissolved in the mixed solvent of chloroform and N,N-dimethylformamide (DMF), and described chloroform and N,N- The volume ratio of dimethylformamide is 9:1, and the polylactic acid solution that makes concentration is 8% (w / v) is used as electrospinning sheath solution;
[0041] (2) Polyvinylpyrrolidone (PVP, weight-average molecular weight Mw is 1300000) is dissolved in the mixed solvent of dehydrated alcohol and DMF, and the volume ratio of described dehydrated alcohol and DMF is 1:1, makes concentration be 4 % (w / v) polyvinylpyrrolidone solution;
[0042] (3) Add the ibuprofen bulk drug to the polyvinylpyrrolidone solution of step (2), the addition of the ibuprofen bulk drug is 20% by mass of the polyvinylpyrrolidone, and stir magnetically at room temperature until dissolved to obta...
Embodiment 2
[0046] A preparation method of ibuprofen transdermal slow-release preparation, the steps are as follows:
[0047] (1) Polylactic acid (PLA, viscosity average molecular weight Mv is 10 0000) is dissolved in the mixed solvent of chloroform and N,N-dimethylformamide (DMF), and described chloroform and N,N- The volume ratio of dimethylformamide is 10:1, and the polylactic acid solution that makes concentration is 6% (w / v) is used as electrospinning sheath solution;
[0048] (2) Polyvinylpyrrolidone (PVP, weight-average molecular weight Mw is 1300000) is dissolved in the mixed solvent of dehydrated alcohol and DMF, and the volume ratio of described dehydrated alcohol and DMF is 2: 1, makes concentration be 6 % (w / v) polyvinylpyrrolidone solution;
[0049] (3) Add the ibuprofen bulk drug to the polyvinylpyrrolidone solution in step (2), the addition of the ibuprofen bulk drug is 15% by mass of the polyvinylpyrrolidone, and stir magnetically at room temperature until dissolved to ob...
Embodiment 3
[0053] A preparation method of ibuprofen transdermal slow-release preparation, the steps are as follows:
[0054] (1) Polylactic acid (PLA, viscosity average molecular weight Mv is 120000) is dissolved in the mixed solvent of chloroform and N,N-dimethylformamide (DMF), and described chloroform and N,N- The volume ratio of dimethylformamide is 8:1, and the polylactic acid solution that makes concentration is 8% (w / v) is used as electrospinning sheath solution;
[0055] (2) Polyvinylpyrrolidone (PVP, weight-average molecular weight Mw is 1000000) is dissolved in the mixed solvent of dehydrated alcohol and DMF, and the volume ratio of described dehydrated alcohol and DMF is 3: 1, and making concentration is 8 % (w / v) polyvinylpyrrolidone solution;
[0056] (3) Add the ibuprofen bulk drug to the polyvinylpyrrolidone solution in step (2), the addition of the ibuprofen bulk drug is 30% by mass of the polyvinylpyrrolidone, stir magnetically at room temperature until dissolved to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com