Chicken infectious anemia subunit vaccine
A chicken infectious anemia, subunit vaccine technology, applied in vaccines, veterinary vaccines, single-stranded DNA viruses, etc. The effect of preventing infection and improving the level of immune antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the screening of chicken infectious anemia VP1, VP2 protein
[0018] In 2016, typical chicken infectious anemia symptoms appeared in a chicken farm in Shandong Province. In order to isolate the pathogen, aseptically collect the thymus of the diseased chicken, homogenize it with sterile saline to make a suspension, centrifuge to collect the cleared bacteria, and then inoculate 6.5-day-old SPF embryos through the allantoic cavity, hatch for 264 hours, and collect the embryo body tissue , after homogenization, repeated freezing and thawing, take the supernatant and freeze it. After the harvested virus liquid was purified, the analysis and detection of virus characteristics in terms of virus content, immunogenicity, specificity and purity were carried out. The results showed that the isolated virus strain had a specific reaction with chicken infectious anemia and had no bacteria or mycoplasma. and exogenous virus contamination.
[0019] The character of the ...
Embodiment 2
[0023] Embodiment 2, the construction of the recombinant baculovirus expressing VP1, VP2 gene
[0024] 2.1 Cutting and optimization of VP1 and VP2 genes
[0025] Cut off 32aa at the N-terminus of the VP1 gene, cut off 7aa at the C-terminus, and mutate the 173rd M to P, and the 304th W to S in the conserved sequence, so as to help the protein to better fold into a tertiary structure, To better expose its antigenic site, add a start codon to the N-terminal; the sequence of the amino acid after optimization and modification is SEQ ID NO: 5, which can well express the antigenic site; at the same time, the codon Optimized for better expression; the nucleotide sequence of the optimized VP1 gene is SEQ ID NO:6. The VP2 gene was codon-optimized, and the nucleotide sequence of the optimized gene is SEQ ID NO:7.
[0026] 2.2 Construction of VP1 positive plasmid
[0027] 2.2.1 Enzyme digestion reaction
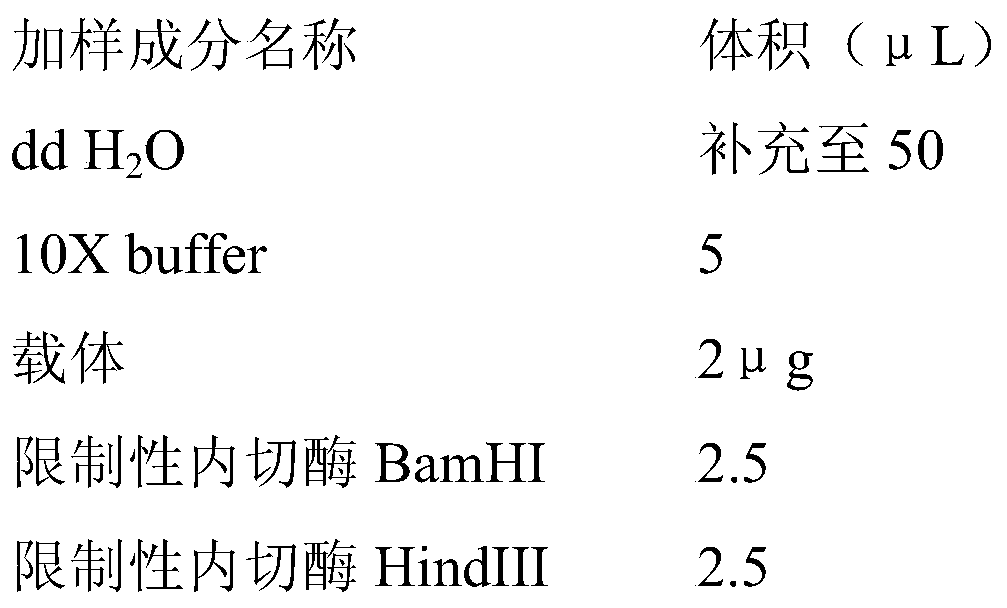
[0028] 2.2.1.1 Mark the 1.5mL EP tube to be used, and add and mix the sample acc...
Embodiment 3
[0097] Embodiment 3: the packing of baculovirus
[0098] (1) Preparation: UV sterilization in a biosafety cabinet for 30 minutes; TNM-FH culture solution was placed in a 27°C water bath and preheated to 27°C.
[0099] (2) Add 2 μg of recombinant DNA to 100 μl of TNM-FH medium without serum and double antibody, and mix well. Add 9 μl Cellfectin Reagent to 100 μl TNM-FH medium without serum and double antibody, and mix well. The liposomes were mixed with the recombinant DNA and allowed to stand at room temperature for 40 min.
[0100] (3) Take out the 6-well plate cells from the incubator at 27°C, discard the supernatant medium, wash the cells three times with pre-warmed TNM-FH culture medium, and discard the TNM-FH culture medium.
[0101] (4) Add 2 ml of 10% fetal bovine serum TNM-FH culture solution to each cell well.
[0102] (5) Gently add the mixture of recombinant DNA and liposomes into each well of cells, mix gently, and culture statically at 27°C for 5-6h.
[0103] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com