Expression optimization and purification method of helicobacter pylori serine proteinase
A technology of serine protease and Helicobacter pylori, which is applied in the field of genetic engineering, achieves good application prospects, high-efficiency solution, and improved soluble expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Contains the construction of the E.coil BL21 (DE3) engineered bacterium of recombinant vector pET-22b-HtrA-His
[0033] Including the following steps:
[0034] 1. According to the recombinant vector pET-22b-HtrA (patent CN108531468A) obtained in the early stage of the laboratory, Sangon Bioengineering (Shanghai) Co., Ltd. was entrusted to perform site-directed mutation of the HtrA gene fragment in the existing plasmid , and then add a 6×His tag (CATCATCACCATCACCAT) to the C-terminus of the gene fragment to obtain a recombinant serine protease gene fragment HtrA-His containing a histidine tag.
[0035] 2. Using pET-22b-HtrA as the expression vector and BL21 as the expression host, insert the recombinant protein gene fragment HtrA-His between the 5'Nde I and 3'Sal I of the multi-cloning site restriction site, and then successfully connect The expression vector is introduced into the host, and the E.coil BL21 engineering bacteria containing the recombinant ve...
Embodiment 2
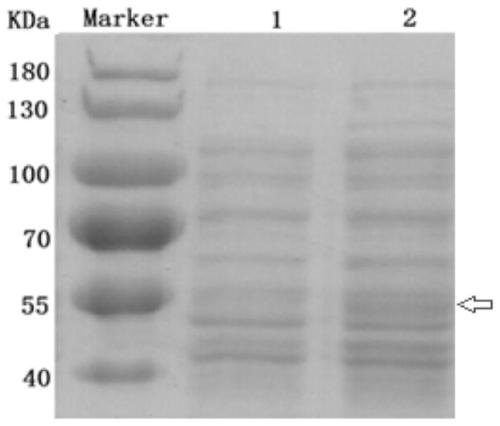
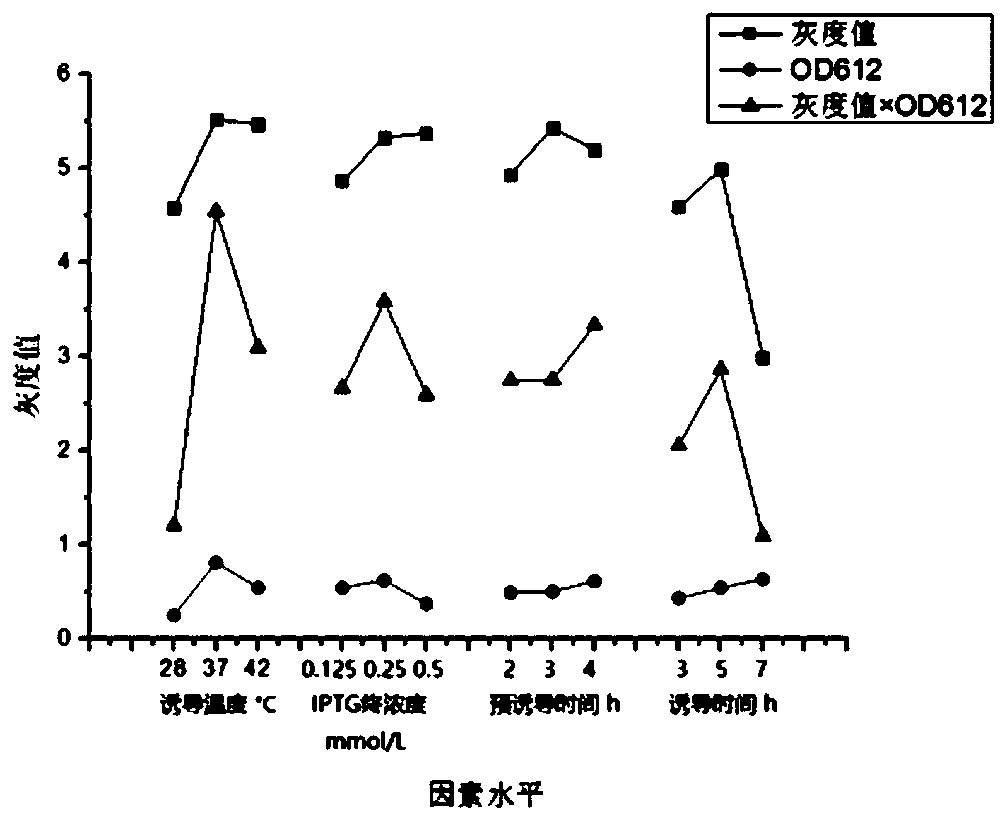
[0037] Example 2 Expression of E.coil BL21 (DE3) Engineering Bacteria Containing Purpose Recombinant Vector pET-22b-HtrA-His and Optimization of Expression Conditions
[0038] 1. Activation and preservation of bacteria
[0039] The original strains of E.coil BL21 engineering bacteria obtained in Example 1 were taken out for activation, single clones were selected and stored in glycerol tubes to ensure the purity and reliability of the experimental strains, including the following steps:
[0040] 1. Relevant culture medium preparation
[0041] LB liquid medium (1L formula): tryptone 10g, yeast extract 5g, sodium chloride 10g;
[0042] LB solid medium (1L formula): tryptone 10g, yeast extract 5g, sodium chloride 10g, agar powder 15g.
[0043] 2. Preparation of solid LB plates
[0044] (1) Ingredients for solid LB medium, sterilized at 121°C for 30 minutes;
[0045] (2) The ultra-clean workbench is sterilized by ultraviolet light for 30 minutes, and the alcohol lamp is lit in t...
Embodiment 3
[0089] The purification method of embodiment 3 recombinant Helicobacter pylori serine protease
[0090] In this example, by adjusting the elution gradient and elution system, the optimal elution conditions for the target protein in affinity chromatography are explored and determined, which specifically includes the following steps:
[0091] 1. Acquisition of purified sample protein solution
[0092] According to the optimal induction expression conditions of the above-mentioned engineering bacteria, a large amount of wet bacteria was obtained and lysed by repeated freezing and thawing methods; after being fully lysed, centrifuged at 4°C and 10,000 rpm for 10 minutes, discarded the precipitate, and collected the supernatant, which was Desired purification load sample protein solution.
[0093] 2. Packing and leak detection of affinity chromatography column
[0094] Put about 1mL of nickel column packing into the column by wet packing method, and at the same time turn on the n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com