A method for selectively leaching and separating vanadium and iron from stone coal vanadium ore
A stone coal vanadium and selectivity technology, applied in the direction of improving process efficiency, can solve the problems of incomplete separation of vanadium and iron, the leaching rate of vanadium needs to be improved, and increase energy consumption, so as to reduce the difficulty of subsequent impurity removal, iron vanadium High separation efficiency and reduced leaching difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
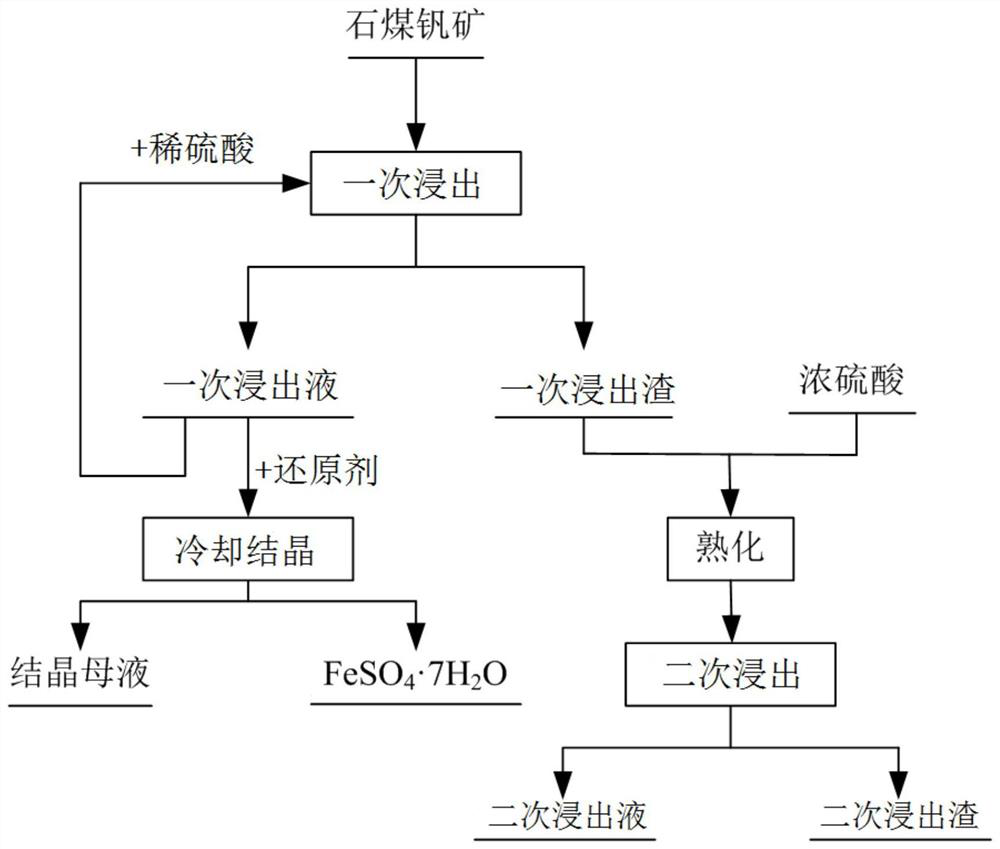
[0061] This embodiment provides a method for selectively leaching and separating vanadium and iron from stone coal vanadium ore, the process flow diagram of the method is as follows figure 1 shown, including the following steps:
[0062] (1) The stone coal vanadium ore is crushed and ball milled to obtain a stone coal vanadium ore powder with a particle size of 80 meshes. The mineral powder is mixed with dilute sulfuric acid with a concentration of 20wt% and then leached once. The solid ratio is 1.5mL / g, the temperature is 90°C, and the time is 1h, and the first leachate and the first leach residue are obtained by filtration;
[0063] (2) add dilute sulfuric acid to the first leaching solution that step (1) obtains in time for recycling, then add iron powder, and Fe 3+ reduced to Fe 2+ Post-cooling and crystallization to recover FeSO 4 ·7H 2 O crystals, the cooling crystallization temperature is 5°C, and the remaining crystallization mother liquor is used for vanadium reco...
Embodiment 2
[0069] This embodiment provides a method for selectively leaching and separating vanadium and iron from stone coal vanadium ore, said method comprising the following steps:
[0070] (1) Crushing and ball milling the stone coal vanadium ore to obtain the stone coal vanadium ore ore powder with a particle size of 120 meshes. The ore powder is mixed with dilute sulfuric acid with a concentration of 10wt% and then leached once. The solid ratio is 6mL / g, the temperature is 60°C, and the time is 3h, and the first leaching solution and the first leaching residue are obtained by filtration;
[0071] (2) add dilute sulfuric acid to the first leaching solution that step (1) obtains in time for recycling, then add potassium thiosulfate, and Fe 3+ reduced to Fe 2+ Post-cooling and crystallization to recover FeSO 4 ·7H 2 O crystals, the cooling crystallization temperature is 10°C, and the remaining crystallization mother liquor is used for vanadium recovery;
[0072] (3) Mix the first ...
Embodiment 3
[0077] This embodiment provides a method for selectively leaching and separating vanadium and iron from stone coal vanadium ore, said method comprising the following steps:
[0078] (1) The stone coal vanadium ore is crushed and ball milled to obtain a stone coal vanadium ore powder with a particle size of 40 meshes. The mineral powder is mixed with dilute sulfuric acid with a concentration of 30wt% and then leached once. The solid ratio is 1mL / g, the temperature is 20°C, and the time is 10h, and the first leaching solution and the first leaching residue are obtained by filtration;
[0079] (2) add dilute sulfuric acid to the first leaching solution that step (1) obtains in time for recycling, then add sodium thiosulfate, and Fe 3+ reduced to Fe 2+ Post-cooling and crystallization to recover FeSO 4 ·7H 2 O crystal, the temperature of cooling and crystallization is 15°C, and the remaining crystallization mother liquor is used for recovery of vanadium;
[0080] (3) Mix the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com