Lithium iron phosphate composite positive electrode material and preparation method and application thereof
A composite cathode material, lithium iron phosphate technology, applied in the field of lithium ion batteries, can solve the problems of poor lithium ion diffusion ability, poor rate performance, affecting the rate performance of lithium iron phosphate materials, etc., to achieve superior charge and discharge rate performance, high capacity retention rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
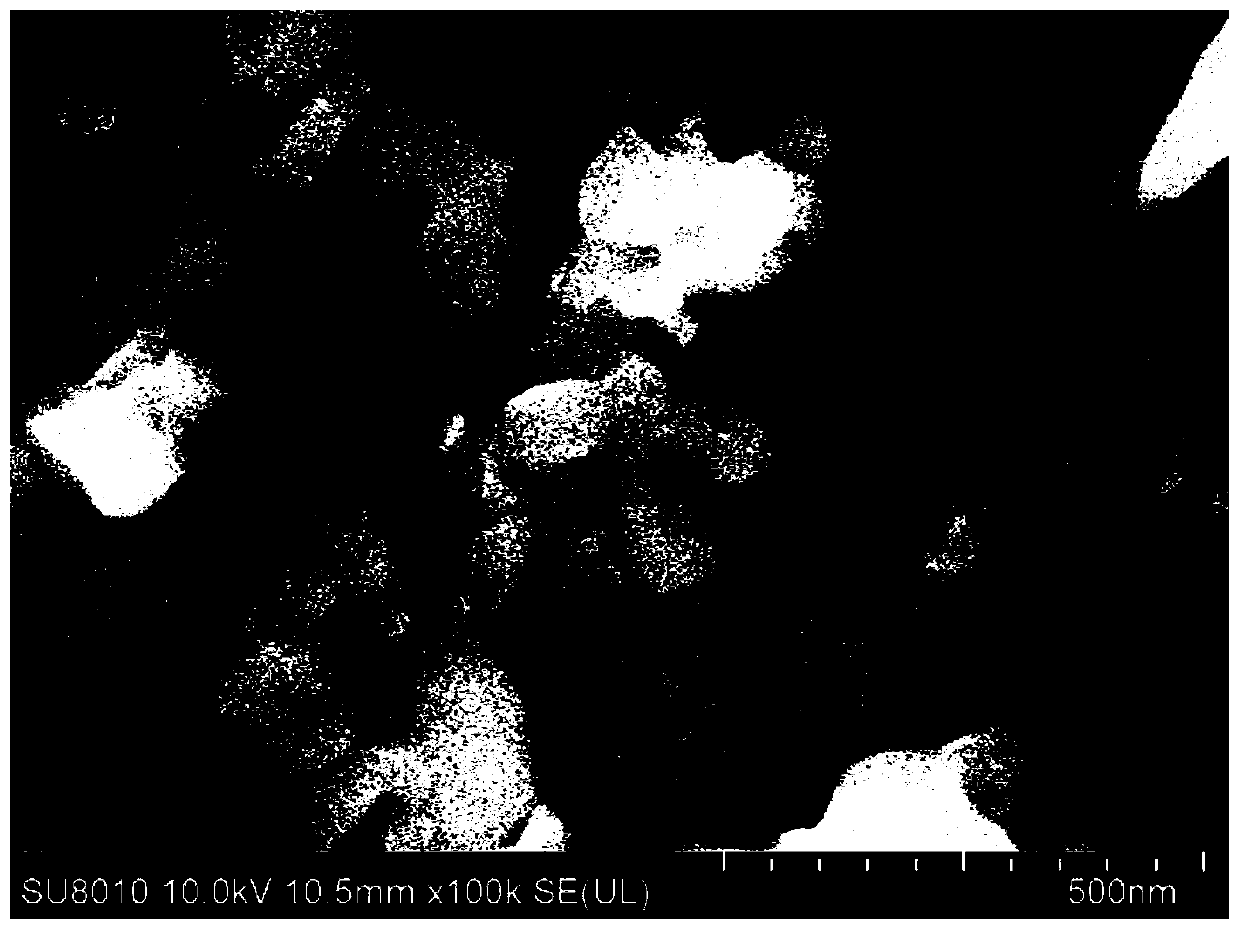
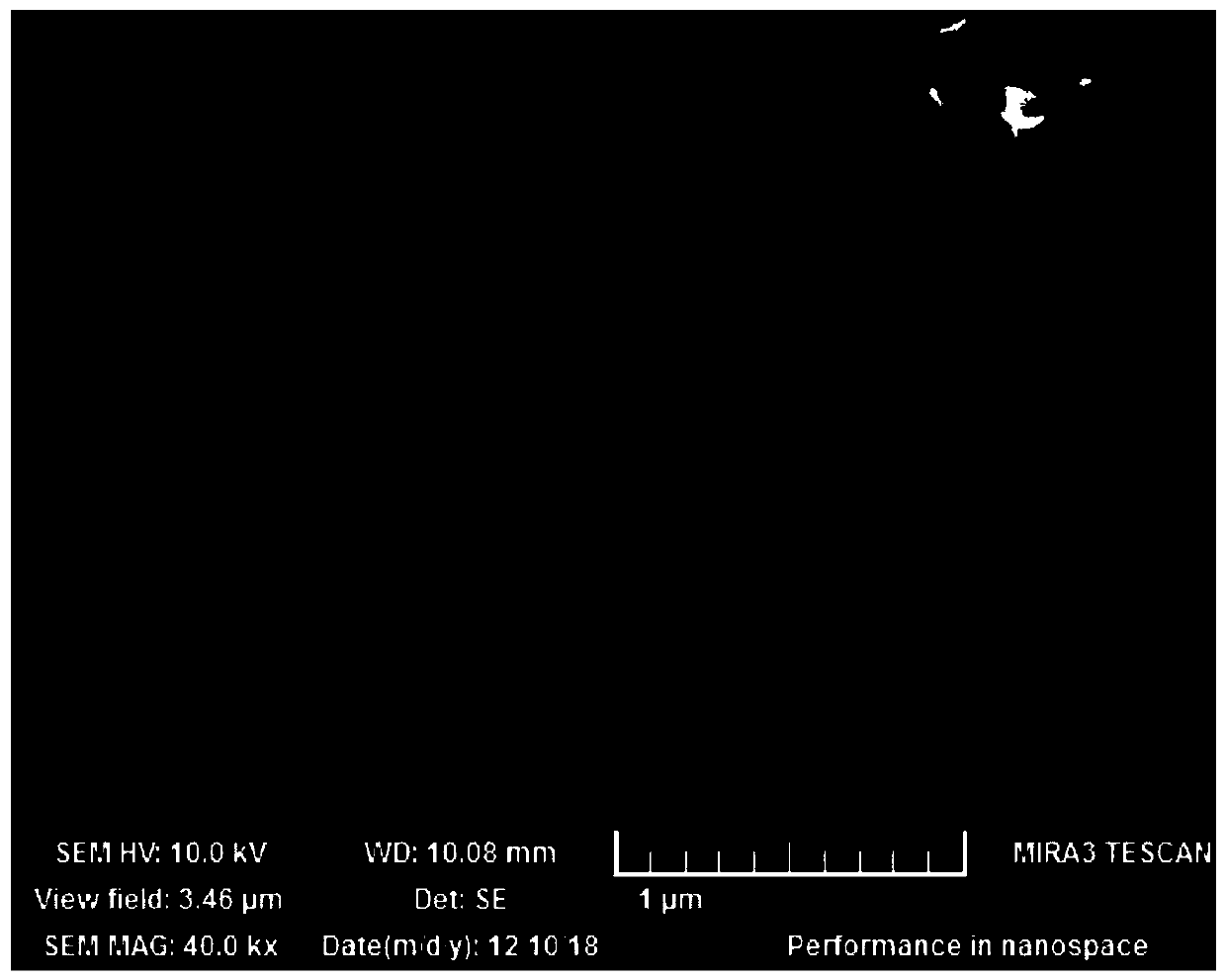
Image
Examples
preparation example Construction
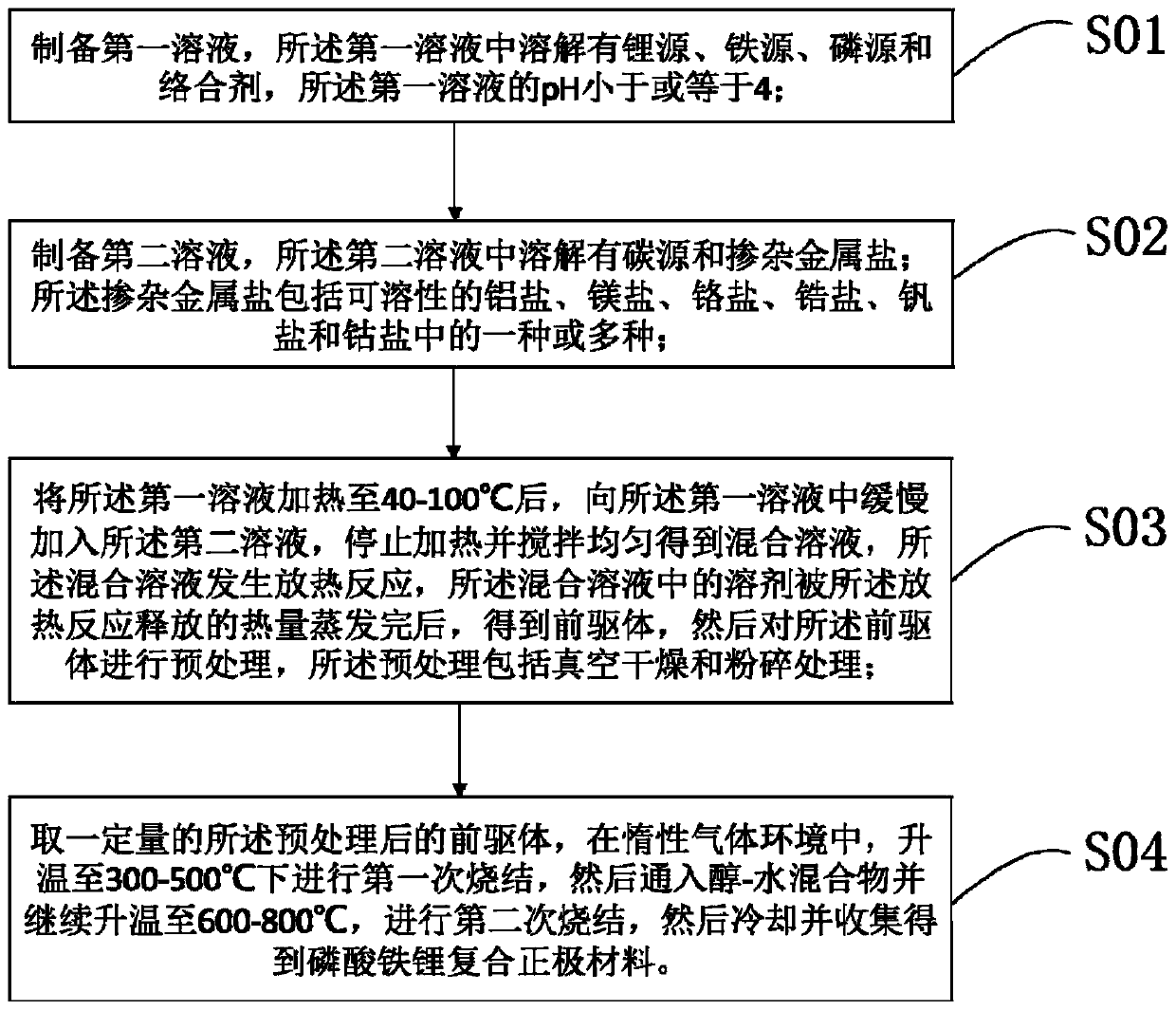
[0070] Such as figure 1 As mentioned, in one embodiment of the present invention, a method for preparing a lithium iron phosphate composite cathode material is provided, which includes the following steps:
[0071] S01: Prepare a first solution in which a lithium source, an iron source, a phosphorus source and a complexing agent are dissolved, and the pH of the first solution is less than or equal to 4;
[0072] S02, prepare a second solution in which a carbon source and a doping metal salt are dissolved; the doping metal salt includes soluble aluminum salt, magnesium salt, chromium salt, zirconium salt, vanadium salt and cobalt salt. One or more of
[0073] S03: After heating the first solution to 40-100°C, slowly add the second solution to the first solution, stop heating and stir evenly to obtain a mixed solution, the mixed solution undergoes an exothermic reaction, so After the solvent in the mixed solution is evaporated by the heat released by the exothermic reaction, a precurs...
Embodiment 1
[0078] A method for preparing lithium iron phosphate composite cathode material includes the following steps:
[0079] (1) Lithium carbonate, ferric nitrate and ammonium dihydrogen phosphate with a molar ratio of 1:1:1 are added to the nitric acid solution, and a citric acid complexing agent is added to adjust the pH to 3 to prepare the first solution.
[0080] (2) Weigh soluble ammonium metavanadate, cobalt nitrate, etc. with a molar percentage of about 0.5% into deionized water to dissolve, and then add a carbon source composed of glucose and fructose, where the mass of the carbon source accounts for lithium carbonate, 25% of the total mass of ferric nitrate and ammonium dihydrogen phosphate, mix well and form the second solution; the molar ratio of ammonium metavanadate to cobalt nitrate is 2:1; the volume ratio of the second solution to the first solution It is 1:3.
[0081] (3) Turn on the heating, heat the first solution to 80°C, slowly pour the second solution into the first ...
Embodiment 2
[0085] A method for preparing lithium iron phosphate composite cathode material includes the following steps:
[0086] Same as Example 1, except that the mole percentage of doped metal salt is increased to 2%. Except for this, the other steps are the same as Example 1, specifically including:
[0087] (1) Add lithium carbonate, ferric nitrate and ammonium dihydrogen phosphate in a molar ratio of 1:1:1 to the nitric acid solution, and add a citric acid complexing agent to adjust the pH to 3 to prepare the first solution.
[0088] (2) Weigh soluble ammonium metavanadate, cobalt nitrate, etc. with a molar percentage of about 2% into deionized water for dissolution, and then add a carbon source composed of glucose and fructose, where the mass of the carbon source accounts for lithium carbonate, 25% of the total mass of ferric nitrate and ammonium dihydrogen phosphate, mix well and form the second solution; the molar ratio of ammonium metavanadate to cobalt nitrate is 2:1; the volume rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com