Preparation and purification methods of fluticasone furoate bulk drug impurity
A technology of fluticasone furoate and purification method is applied in the field of preparation and purification of fluticasone furoate crude drug impurities, can solve the problem of not finding fluticasone furoate crude drug impurities and the like, and achieves easy purification, ensures accuracy, and improves quality standards. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
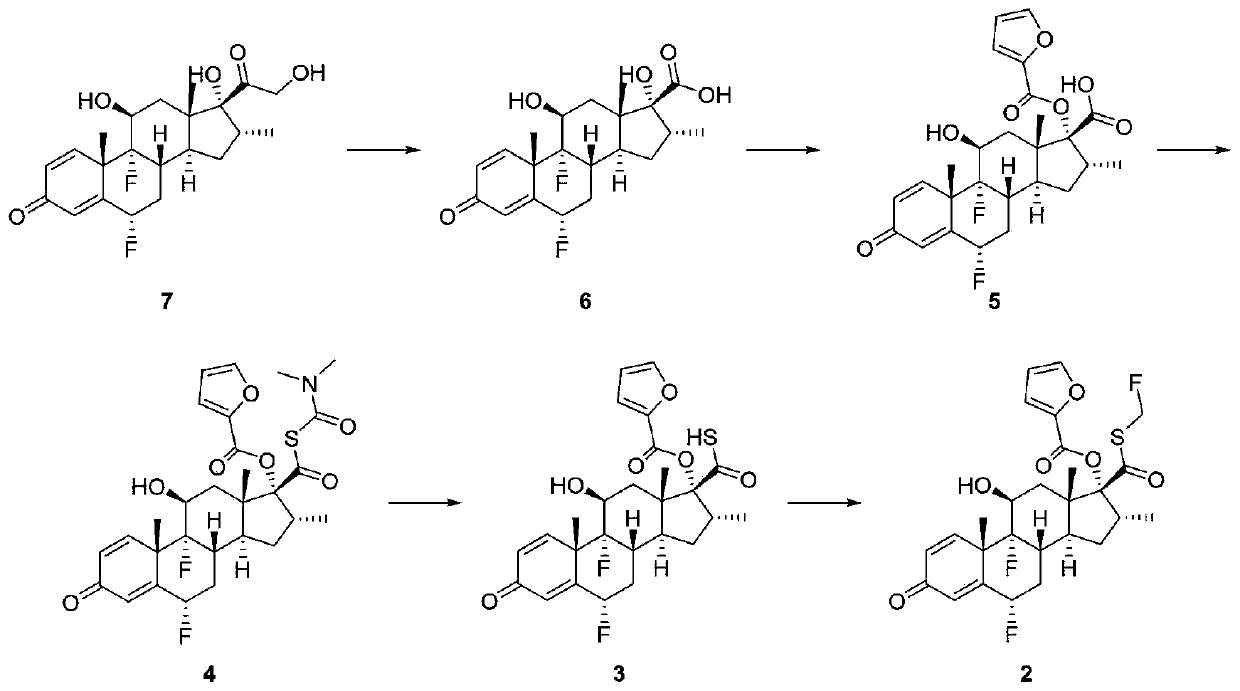
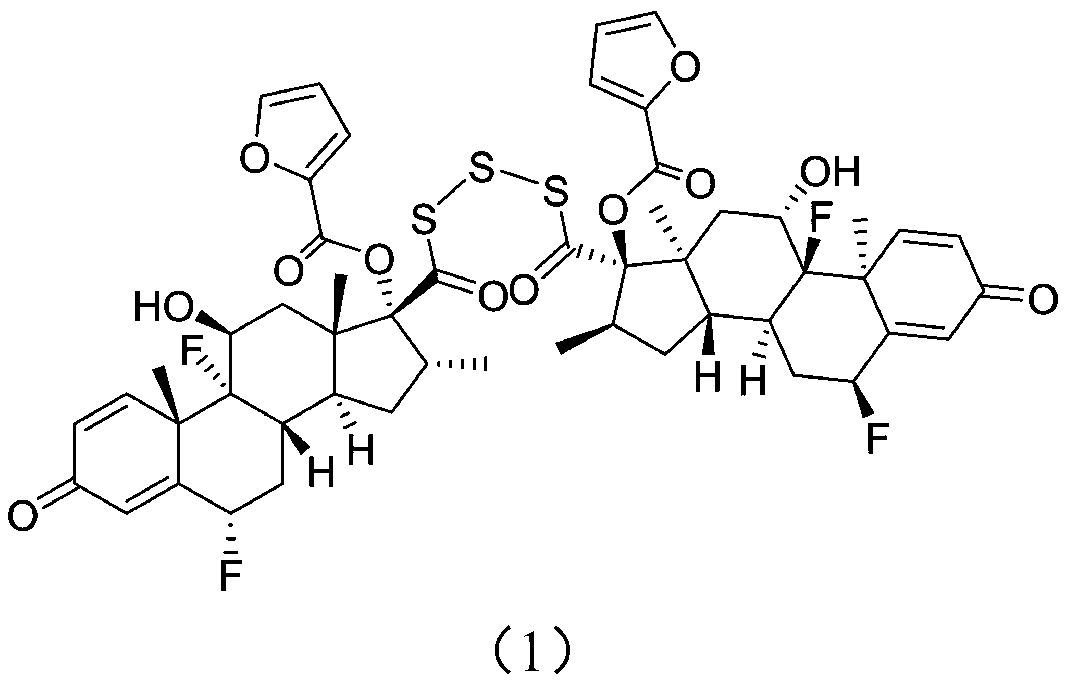
[0031] The present invention provides a kind of preparation method of fluticasone furoate crude drug impurity, described fluticasone furoate crude drug impurity has the structure of following formula (1):
[0032]
[0033] figure 1 A schematic diagram of the synthetic route of the impurity in the embodiment of the present invention is shown. like figure 1 Shown, the preparation method of described formula (1) fluticasone furoate crude drug impurity comprises:
[0034] With compound 3(6α,9α-difluoro-17α-[(2-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl-3-oxo-androst-1,4-diene-17β -thiocarboxylic acid) as a raw material, in the presence of a base, protonated or non-protonated solvent, reacts with sulfur chloride to generate a mixture containing compound 1; wherein compound 1 is the generated impurity.
[0035] Specifically, the base includes sodium carbonate, potassium carbonate, cesium carbonate, sodium phosphate, potassium phosphate, 4-dimethylaminopyridine, triethylamine, N...
Embodiment 1
[0053] The method for the preparation of fluticasone furoate crude drug impurities is specifically:
[0054] At room temperature, the intermediate compound 3 (1.2g, 2.4mmol) that generates fluticasone furoate was added to a 100mL three-necked flask, and 25mL of tetrahydrofuran and N-methylmorpholine (0.24g, 2.4mmol) were added, followed by nitrogen gas Protection, the use of nitrogen protection is to extract the air inside the container and fill it with nitrogen gas. The main purpose is to prevent the flammable gas in the container from forming a flammable mixture with the oxygen in the air, which may cause internal combustion or explosion; nitrogen protection is used Nitrogen replacement device.
[0055] After the operation, the temperature was lowered to 0°C, and sulfur chloride (0.16g, 1.2mmol) was weighed and slowly added dropwise. After the addition was completed, it was kept and stirred for 0.5h, and then placed in an ice-water bath, and 25mL of saturated saline was adde...
Embodiment 2
[0057] The preparation method of fluticasone furoate crude drug impurity is specifically:
[0058] Under room temperature conditions, the intermediate 3 (3.1g, 6.1mmol) that will generate fluticasone furoate was added to a 250mL three-necked flask, and 60mL of dichloromethane and triethylamine (1.2g, 6.1mmol) were added, followed by nitrogen protection, The use of nitrogen protection is to extract the air inside the container and fill it with nitrogen gas. The main purpose is to prevent the flammable gas in the container from forming a flammable mixture with the oxygen in the air, which may cause internal combustion or explosion; nitrogen protection uses nitrogen replacement device.
[0059] After the operation, cool down to -20°C, weigh sulfur chloride (0.41g, 3.1mmol) and slowly add it dropwise. After the addition, keep stirring for 20min, then place it in an ice-water bath, add 60mL of water to stir, extract and separate, and the organic phase is decompressed. The residue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com