Optimized high-temperature trehalase MS-Tre capable of being expressed efficiently in aspergillus niger and encoding gene and application of optimized high-temperature trehalase MS-Tre
A trehalase and high-efficiency expression technology, applied in the field of genetic engineering, can solve the problems of low expression level of recombinant trehalase, and achieve the effects of good stability, reduced cooling energy consumption, and improved utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
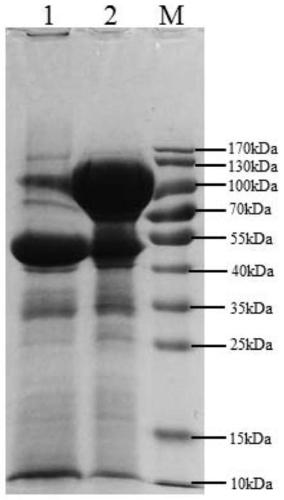
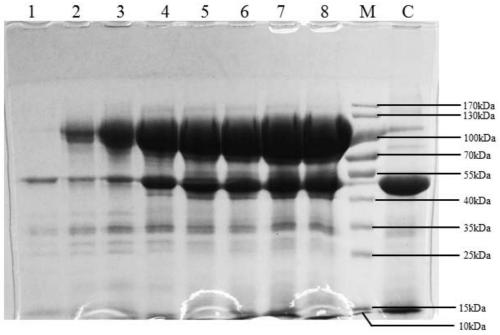
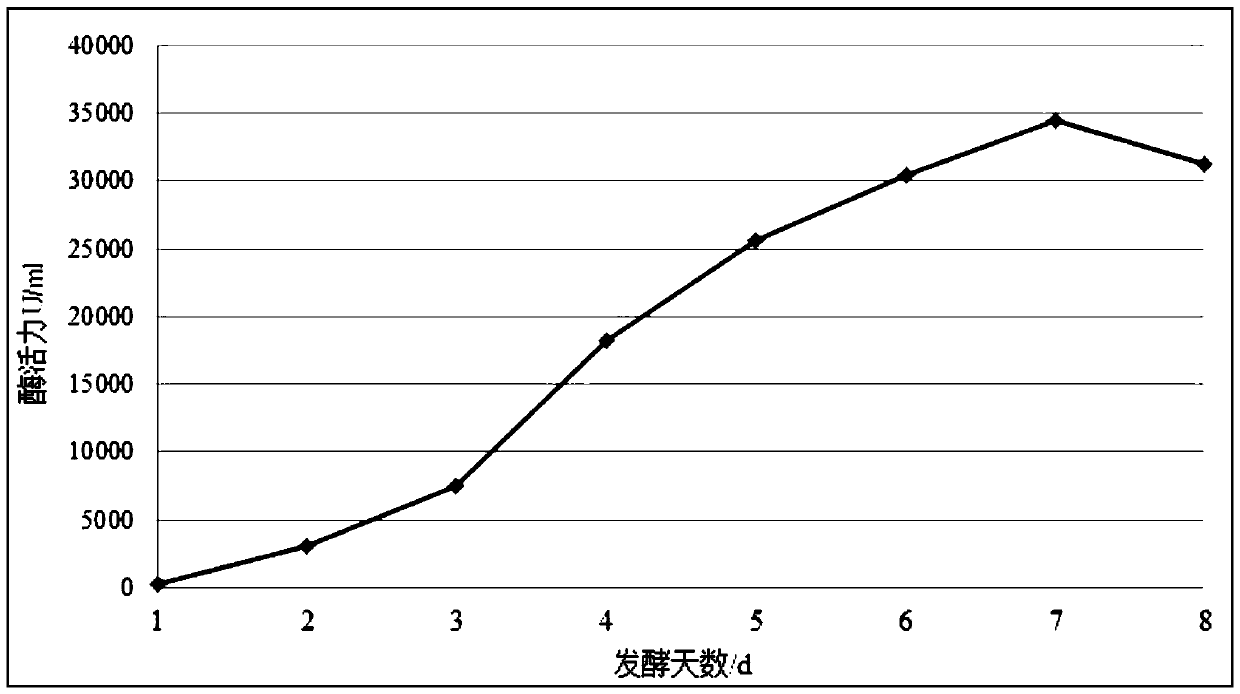
Image
Examples
Embodiment 1
[0046] Embodiment 1, the construction of general expression vector
[0047] Using the Aspergillus niger genome as a template, perform PCR amplification (Prime STAR premix HS (purchased from takara company)), using primer Ttef-fw (as shown in SEQ ID NO.8, Primer means primer, the same below) and primer Ttef-rev (as shown in SEQ ID NO.9) amplifies the tef terminator (as shown in SEQ ID NO.3), using primer amyA-fw (as shown in SEQ ID NO.12) and primer amyA-rev (as shown in SEQ ID NO.12) Shown in SEQ ID NO.13) to amplify the last 1000bp (as shown in SEQ ID NO.5) of the gene encoding neutral amylase amyA, use primer pyrG-fw (as shown in SEQ ID NO.10) as a template with the Aspergillus nidulans genome shown) and primer pyrG-rev (shown in SEQ ID NO.11) to amplify the pyrG marker (shown in SEQ ID NO.4), and the PCR products obtained are respectively named as Ttef, amyA, pyrG (shown in SEQ ID NO. .3, as shown in SEQ ID NO.5, as shown in SEQ ID NO.4), use NEBuilder HiFi DNA Assembly Cl...
Embodiment 2
[0048] Embodiment 2, containing the target gene expression vector construction
[0049]Using the optimized MS-Tre gene nucleotide sequence of the synthetic amino acid sequence, the non-optimized MSre gene nucleotide sequence of the synthetic amino acid sequence, and the Aspergillus niger genome as templates, primer Tre1-fw (such as SEQ ID NO.18 shown) and primer Tre-rev (shown in SEQ ID NO.19) amplified to obtain the MS-Tre gene sequence (shown in SEQ ID NO.2) after amino acid sequence optimization, with primer Tre2-fw (shown in SEQ ID NO.2) ID NO.20) and primer Tre-rev (as shown in SEQ IDNO.19) amplified to obtain the unoptimized MSre gene sequence of amino acid sequence (as shown in SEQ ID NO.2), with primer PamyA-fw (as shown in SEQ ID NO.16) and the primer PamyA-rev (shown in SEQ ID NO.17) were amplified to obtain the Aspergillus niger neutral amylase promoter PamyA sequence (shown in SEQ ID NO.7). The 3 PCR-amplified PCR fragments were connected to the linearized univers...
Embodiment 3
[0050] Embodiment 3, transformation of expression vector pMD20-PamyA-MS-Tre-Ttef-pyrG-amyA and pMD20-PamyA-MSre-Ttef-pyrG-amyA plasmid in Aspergillus niger
[0051] Prepared according to the steps provided in (Gomi K, Iimura Y, Hara S. Integrative transformation of Aspergillusoryzae with a plasma containing the Aspergillus nidulans argB gene [J]. Agricultural and biological chemistry, 1987, 51 (9): 2549-2555.) The protoplasts of the host fungus Aspergillus niger (ΔpyrG), the expression vectors pMD20-PamyA-MS-Tre-Ttef-pyrG-amyA and pMD20-PamyA-MSre-Ttef-pyrG-amyA obtained above were transformed into the protoplasts respectively , coated hypertonic CD medium (containing 1M sucrose, 0.3% (w / v) NaNO 3 , 0.2% (w / v) KCl, 0.05% (w / v) MgSO 4 .7H 2 O, 0.1% (w / v) K 2 HPO 4 .3H 2 O, 0.001% (w / v) FeSO 4 .7H 2 (0, 2% (w / v) agar powder, pH 5.5, the unit of w / v is g / mL), put it into a 30° C. incubator, and observe the growth of transformants after 5 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com