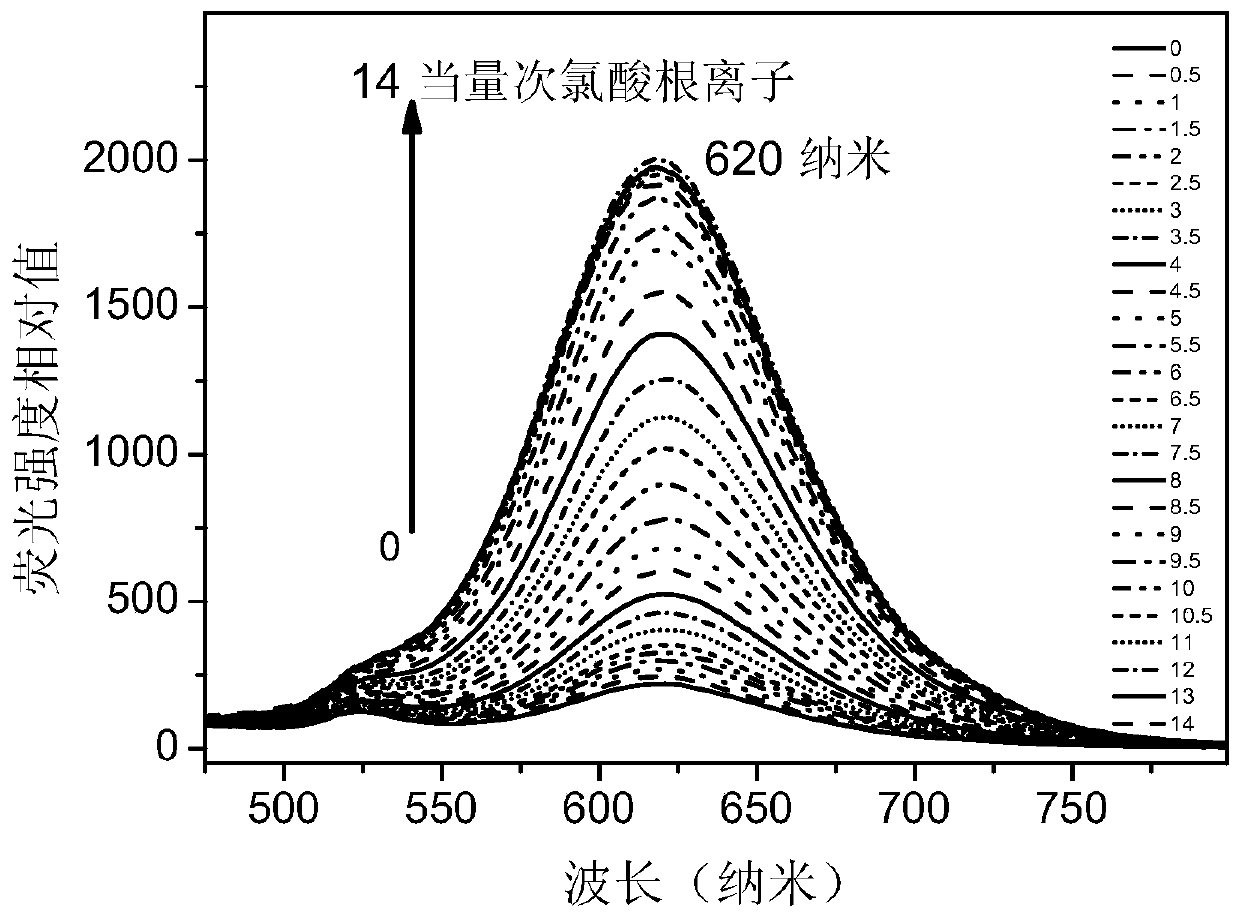
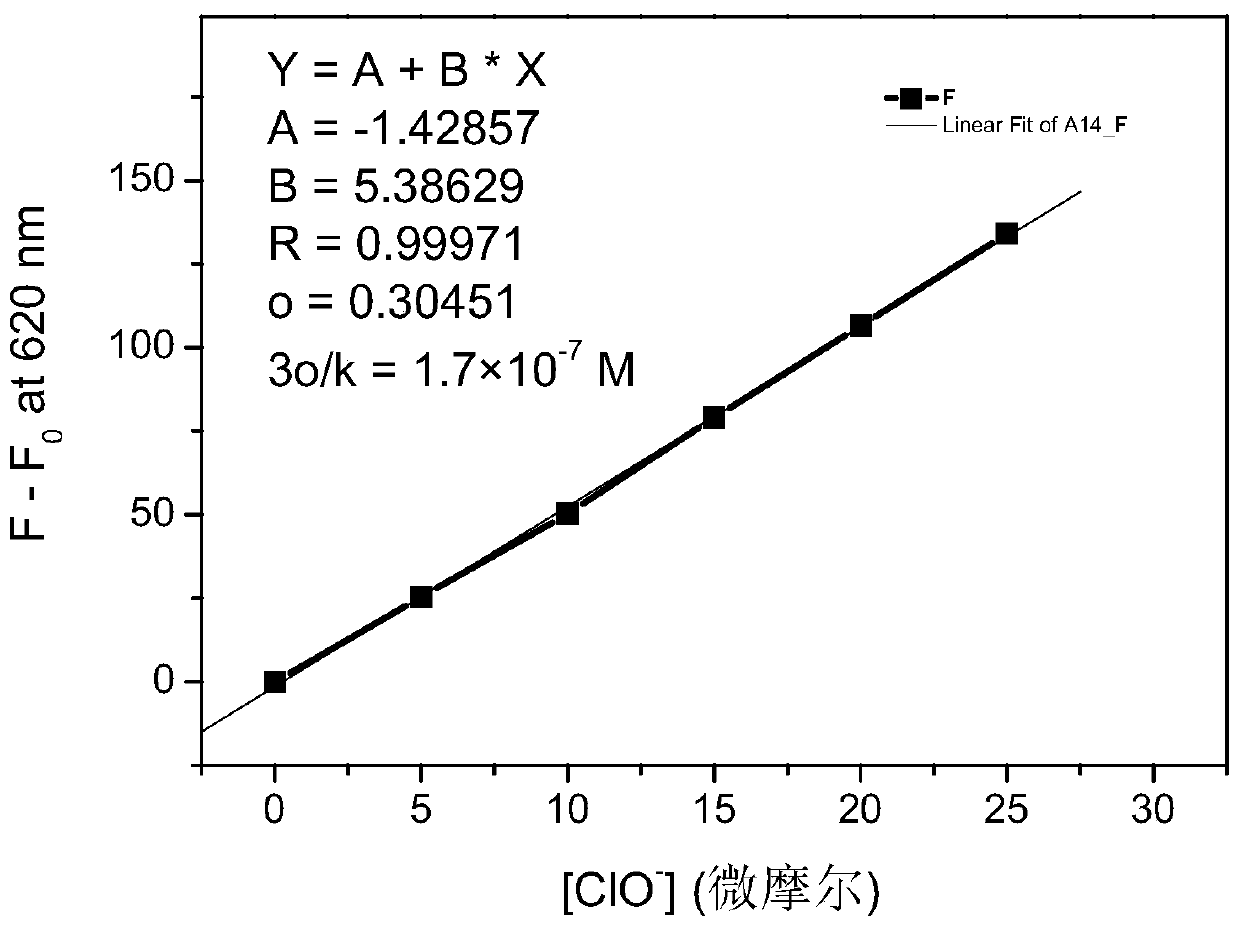
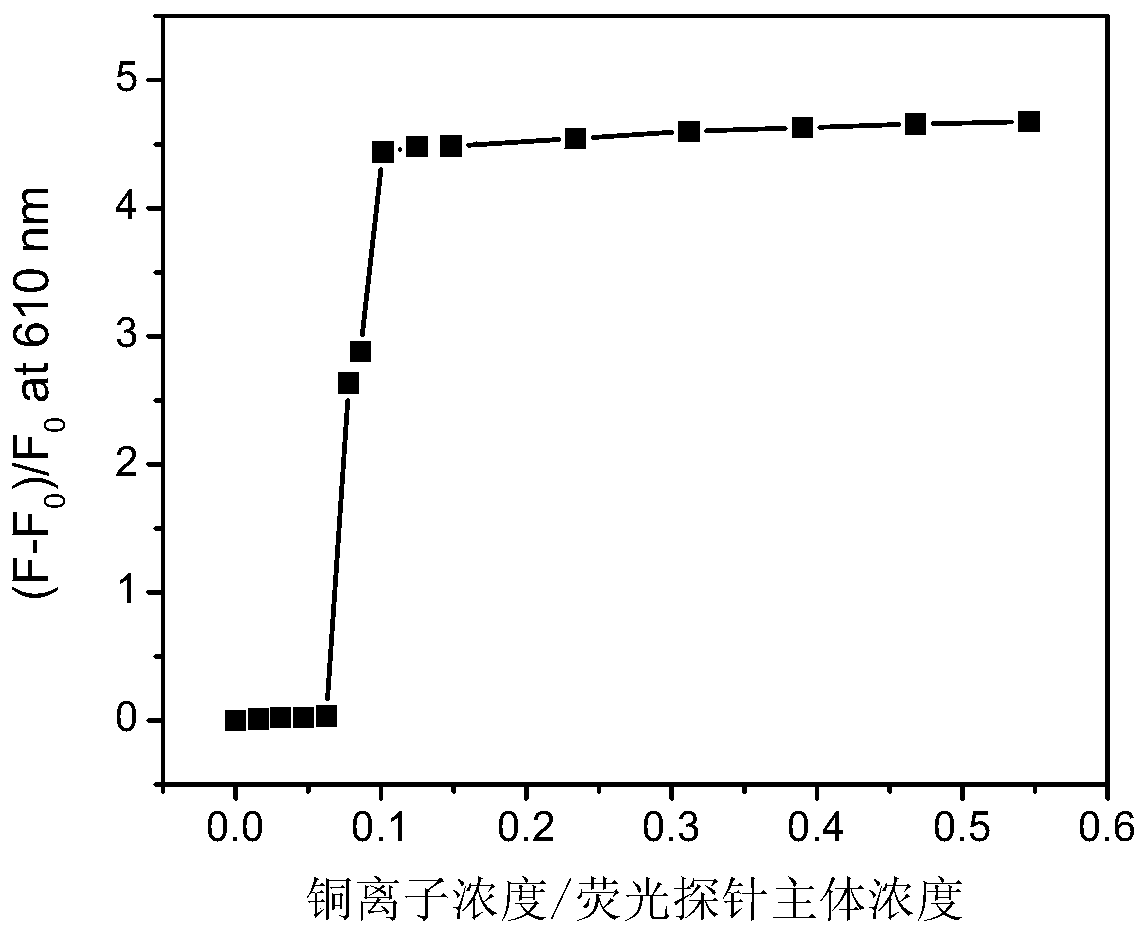
Phenothiazine benzaldehyde derivative and preparation method thereof, and phenothiazine quinoline molecular fluorescent probe and preparation method and application thereof
A technology of phenothiazine quinoline and fluorescent probe, which is applied in the field of phenothiazine benzaldehyde derivatives and its preparation, can solve the problems of lack of fast, effective, low-cost detection methods for environmental pollutants and intracellular active oxides, etc. Achieve the effects of simple and easy preparation method, wide range of pH value and widening of pH application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of phenothiazine benzaldehyde derivative
[0035] Proceed as follows:
[0036]1) Compound 4-N,N-diethylamino-2-hydroxyl-benzaldehyde (387mg, 2mmol) and 10-(6-bromo-hexyl)-10H-phenothiazine (1.08g, 3mmol) were added to In 20 mL of anhydrous acetonitrile, add 53 mg of potassium iodide, then add 550 mg of ground potassium carbonate, stir evenly, and react under reflux for 24 hours in the dark;
[0037] 2) After cooling to room temperature, add potassium tetrafluoroborate to the above reaction solution and stir for 2 hours for anion exchange, distill the obtained crude product solution under reduced pressure and extract with dichloromethane, collect the organic phase, and dry with anhydrous sodium sulfate , and the organic phase was spin-dried and purified by column separation. The eluent is petroleum ether: ethyl acetate (4 / 1, v / v). The purified product was dried in a vacuum oven to obtain 806 mg of oily substance with a yield of 85%.
Embodiment 2
[0038] Embodiment 2, the preparation of phenothiazine quinoline derivatives
[0039] Proceed as follows:
[0040] 1) Mix 4-diethylamino-2-(6-phenothiazin-10-yl-hexyloxy)-benzaldehyde (224mg, 0.5mmol) obtained in the above-mentioned Example 2 with N-ethyl-2 - Add methylquinoline iodonium salt (150 mg, 0.5 mmol) into 10 mL of absolute ethanol, add 200 μL of hexahydropyridine, and heat to reflux reaction in the dark. to 22°C;
[0041] 2) Add 63mg (0.5mmol) potassium tetrafluoroborate (KBF 4 ) and stirred and reacted for 2 hours, after anion exchange, the crude product was obtained;
[0042] 3) The above reaction solution was filtered to remove inorganic salts, the liquid phase was evaporated under reduced pressure to remove the solvent and concentrated to 3 ml, the solid product was recrystallized with 5 ml of absolute ethanol, and after drying, 246 mg of yellow solid product was obtained, which was the prepared 1 -{2-[4-Diethylamino-2-(4-phenothiazine-hexyloxy)-phenyl]-vinyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com