Method for preparing tin-doped nano-amorphous titanium dioxide by using intermediate of chlorination process
A titanium dioxide and nano-amorphous technology, which is applied in the field of photocatalytic nano-new material preparation, can solve the problems of large product particle size, calcination of agglomerated products, pollution of the environment, etc., and achieves the effect of improving performance, reducing agglomeration and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
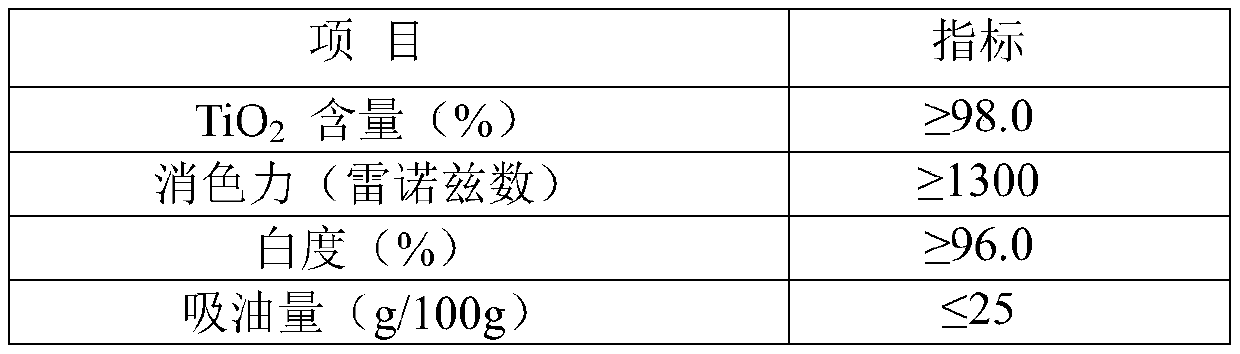
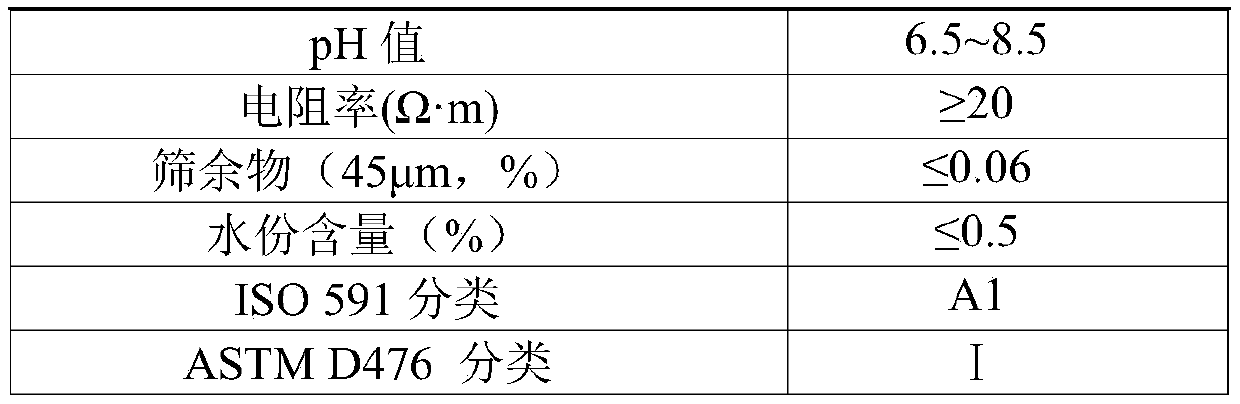
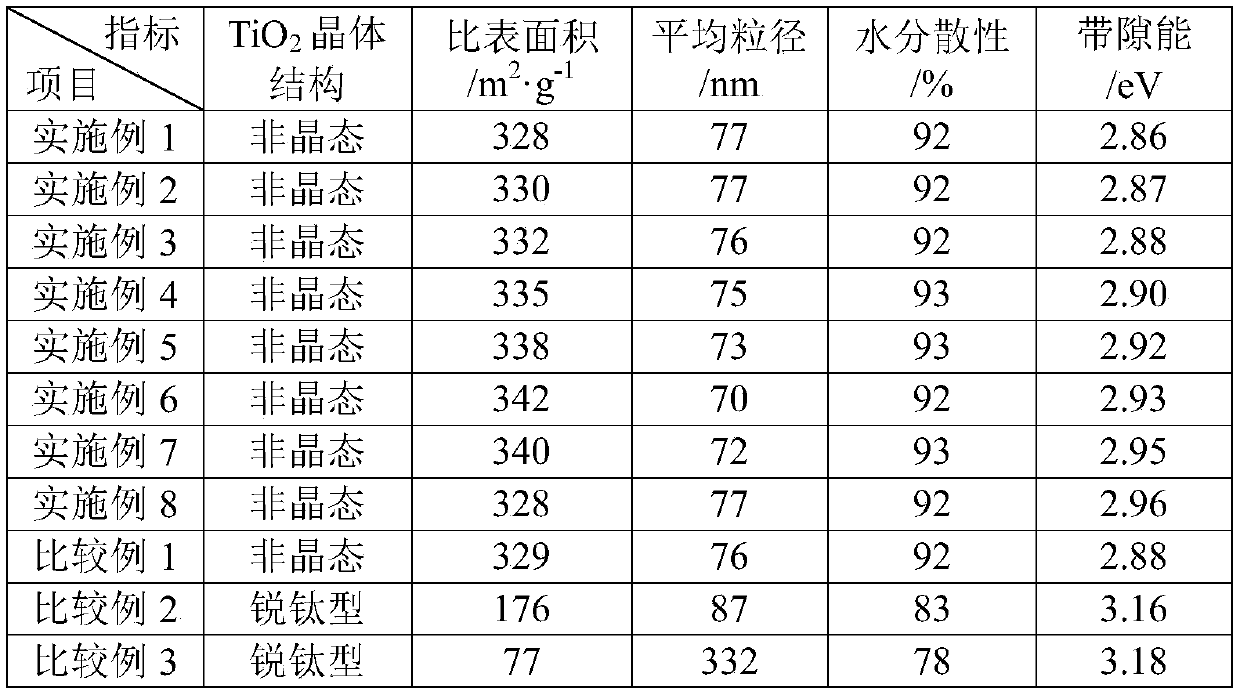
[0035] A, at room temperature, with the peristaltic pump that flow rate is 5mL / min, prepare the intermediate product titanium tetrachloride (TiCl3) of rutile type titanium dioxide through the refined chloride method 4 Mass percent composition is 98.0%), tin tetrachloride is added to 10L NaOH mixed aqueous solution containing sodium dodecylbenzene sulfonate simultaneously with the peristaltic pump of 0.25mL / min (the mass concentration of NaOH 10% and the mass concentration of sodium dodecylbenzenesulfonate is 0.1%), the mol ratio of titanium tetrachloride addition and NaOH is 1:5, and tin tetrachloride addition is 2.0% of the quality of titanium tetrachloride %, while adding, stir at a speed of 3000r / min at a high speed. After the addition is completed, add an aqueous ammonium carbonate solution with a mass concentration of 10%. The ammonium carbonate addition is 0.3% of the mass of titanium tetrachloride, and then the temperature is raised to 71°C , continue to stir at a speed...
Embodiment 2
[0040] A, at room temperature, with the peristaltic pump that flow rate is 5mL / min, prepare the intermediate product titanium tetrachloride (TiCl3) of rutile type titanium dioxide through the refined chloride method 4 Mass percent composition is 98.2%), with the peristaltic pump that flow rate is 0.25mL / min tin tetrachloride is added into 10L NaOH mixed aqueous solution containing sodium dodecylbenzene sulfonate simultaneously (the mass concentration of NaOH 11% and the mass concentration of sodium dodecylbenzene sulfonate is 0.12%), the mol ratio of titanium tetrachloride addition and NaOH is 1:5.2, and tin tetrachloride addition is 2.5% of the quality of titanium tetrachloride %, while adding, stir at a speed of 3100r / min at a high speed. After the addition is complete, add an aqueous ammonium carbonate solution with a mass concentration of 12%. The ammonium carbonate addition is 0.32% of the mass of titanium tetrachloride, and then heat up to 70°C , continue to stir at a sp...
Embodiment 3
[0045] A, at room temperature, with the peristaltic pump that flow rate is 5mL / min, prepare the intermediate product titanium tetrachloride (TiCl3) of rutile type titanium dioxide through the refined chloride method 4 The mass percent composition is 98.3%), tin tetrachloride is added to 10L NaOH mixed aqueous solution containing sodium dodecylbenzenesulfonate (the mass concentration of NaOH 12% and the mass concentration of sodium dodecylbenzenesulfonate is 0.15%), the mol ratio of titanium tetrachloride addition and NaOH is 1:5.5, tin tetrachloride addition is 3.0% of the quality of titanium tetrachloride %, while adding, stir at a speed of 3200r / min at a high speed. After the addition is completed, add an aqueous ammonium carbonate solution with a mass concentration of 14%. The ammonium carbonate addition is 0.35% of the mass of titanium tetrachloride, and then the temperature is raised to 72°C , continue stirring at a speed of 510r / min, ripen for 3.5h, and cool to room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com