A kind of preparation method of r-(-)-atomoxetine hydrochloride
A technology of atomoxetine hydrochloride and mandelic acid salt, which is applied in the field of medicine, can solve the problems of human skin permeability, dizziness symptoms, unpleasant smell, and difficult sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
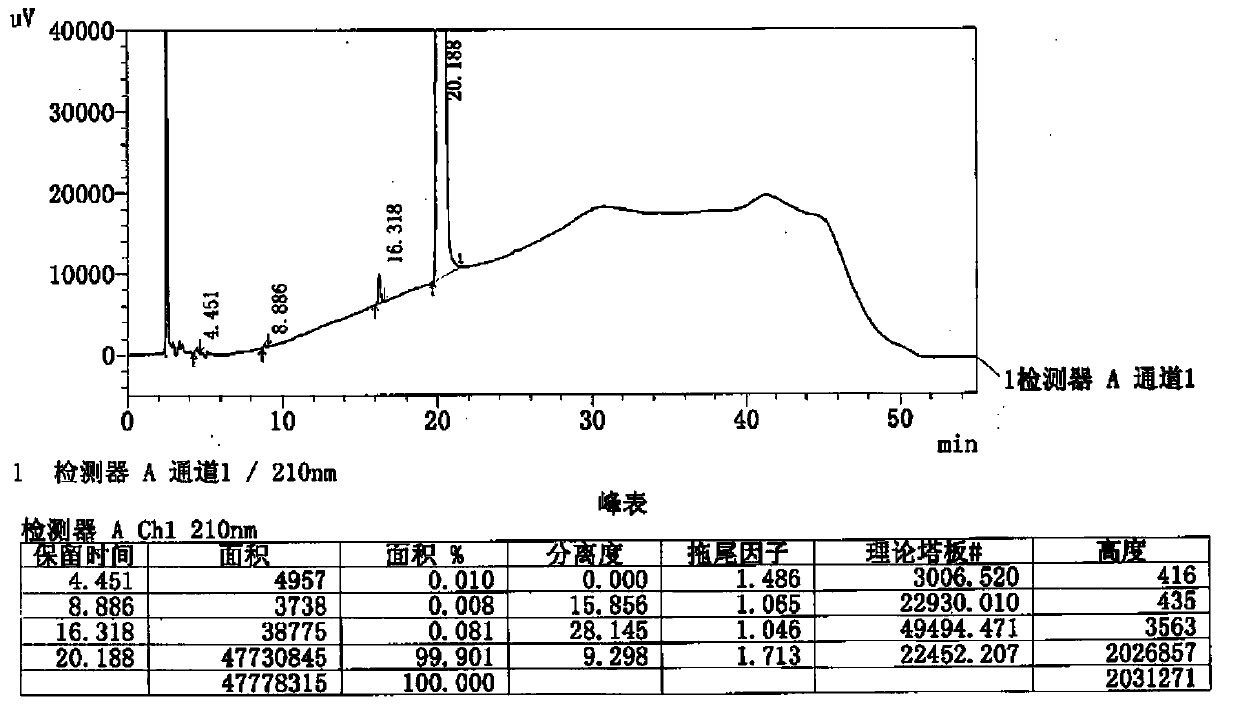
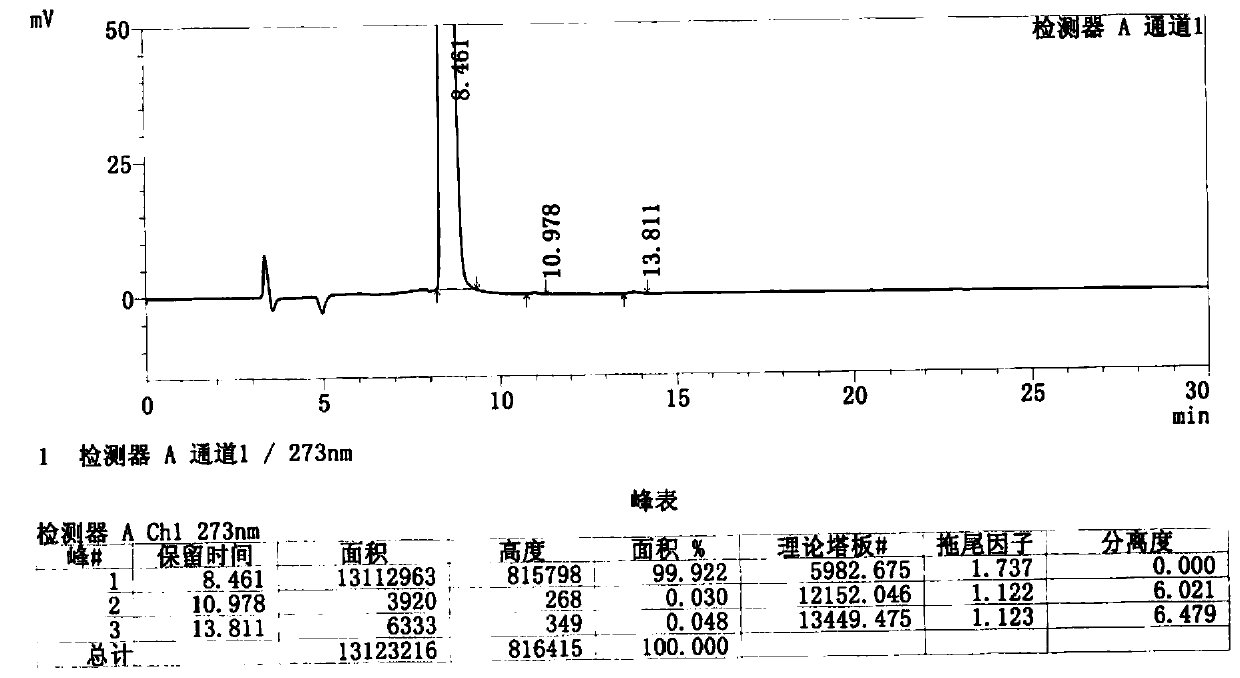
Image
Examples
preparation example Construction
[0073] In an embodiment of the present invention, the preparation method of the R-(-)-atomoxetine hydrochloride comprises the following steps:
[0074] (1) Slowly add compound 1, that is, 1-phenyl-2-propenyl-1-ketone (alcohol solution of methylamine and 1-phenyl-2-propenyl-1-ketone) dropwise in the alcohol solution of methylamine The molar ratio is 1.0-1.5:1, such as 1.2:1, 1.3:1 or 1.4:1), and the temperature is controlled at 20-40°C (such as 25°C or 30°C) to stir the reaction, and the sample is detected until there is no 1 - When phenyl-2-propenyl-1-one remains, add sodium borohydride in batches and control the temperature of the system at -5-5°C (the temperature can be -1-1°C, 0-2°C or -2-0 ℃), the molar ratio of 1-phenyl-2-propenyl-1-one to the total amount of sodium borohydride is 1:1.0-1.2 (the molar ratio can be 1:1.0, 1:1.05, 1:1.08, 1 : 1.2), after the stirring reaction finishes, add purified water, hydrochloric acid, stir, add toluene to extract 2 to several times, ...
Embodiment 1
[0088] Add 1.36 kg of 33% methylamine ethanol solution into the reaction kettle, slowly add 1.58 kg of 1-phenyl-2-propenyl-1-one dropwise under stirring, and stir the reaction at a temperature of 25°C, and take samples to detect when there are no residues of raw and auxiliary materials , add 0.48kg of sodium borohydride in batches, control the temperature of the system at -1~1°C, add 10L of purified water and 2L of 2N hydrochloric acid after the stirring reaction is completed, stir for 30min, add 10L of toluene for extraction twice, combine the organic phases, and add 20L of purified water Wash with water to obtain 3-methylamino-1-phenyl-1-propanol toluene solution for use.
[0089]At room temperature, add 4.4 kg of triethylene glycol dimethyl ether to the reaction kettle containing the 3-methylamino-1-phenyl-1-propanol toluene solution, add 2.39 kg of KOH into the reaction kettle, stir and heat up to When the temperature of the system reaches 120°C, reflux for water separatio...
Embodiment 2
[0094] Add 1.36 kg of 33% methylamine ethanol solution into the reaction kettle, slowly add 1.58 kg of 1-phenyl-2-propenyl-1-one dropwise under stirring, and stir the reaction at a temperature of 25°C, and take samples to detect when there are no residues of raw and auxiliary materials , add 0.49kg of sodium borohydride in batches, control the temperature of the system at 0-2°C, add 10L of purified water and 2L of 2N hydrochloric acid after the stirring reaction is completed, stir for 30min, add 15L of toluene for extraction twice, combine the organic phases, wash with 20L of purified water , to get 3-methylamino-1-phenyl-1-propanol toluene solution, set aside.
[0095] At room temperature, add 6.6 kg of triethylene glycol dimethyl ether to the reaction kettle equipped with 3-methylamino-1-phenyl-1-propanol toluene solution, add 2.39 kg of KOH into the reaction kettle, stir and heat up to When the temperature of the system reaches 125°C, reflux for water separation for 1 hour,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com