Narrowband receptor conjugated polymer with oligoethylene glycol side chain structure, and preparation method and application thereof
A technology of conjugated polymers and polymerization reactions, which is applied to narrow-band acceptor-type conjugated polymers containing oligoethylene glycol side chain structures and their preparation and application fields, and can solve problems such as high toxicity and dangerous halogenated solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
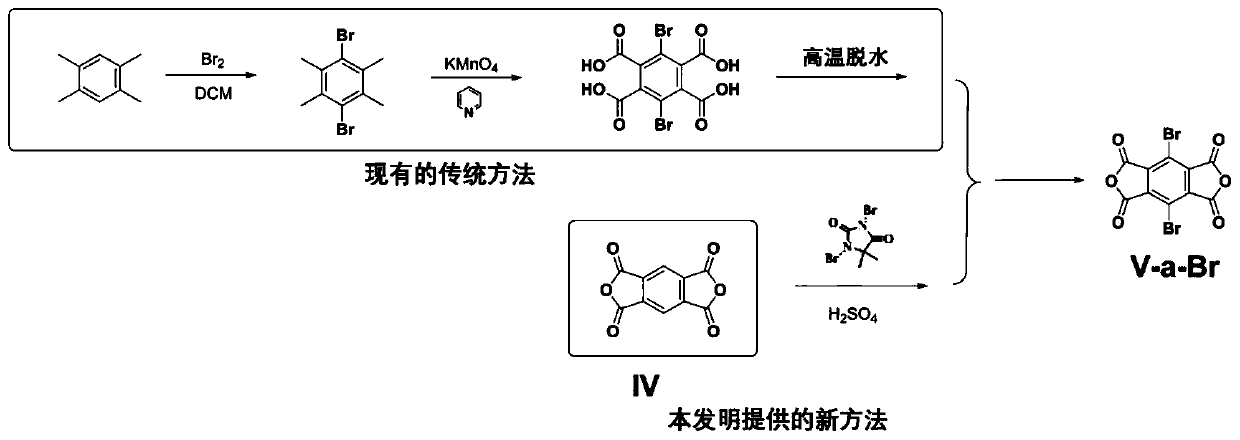
[0142] The invention provides a kind of synthetic method of dihalogenated pyromellitic anhydride, comprising the following steps:
[0143] 1) Under the action of a strong acid, after halogenating the pyromellitic anhydride monomer with the structure of formula (VI), a halogenating agent and a solvent, the dihalogenated pyromellitic anhydride with the structure of formula (V-a) is obtained;
[0144]
[0145] Wherein, G is -Cl, -Br or -I.
[0146] In principle, the present invention has no special restrictions on the specific selection of the strong acid. Those skilled in the art can select and adjust according to the actual situation, performance requirements and product requirements. The present invention is to better ensure the performance of the prepared product and ensure the The conjugated polymer has good solubility and comprehensive properties in green solvents. The strong acid preferably includes concentrated sulfuric acid and / or oleum, more preferably concentrated s...
Embodiment 1
[0253] Synthesis of monomer
[0254] 1) Monomer Q2-NH 2 -3n:
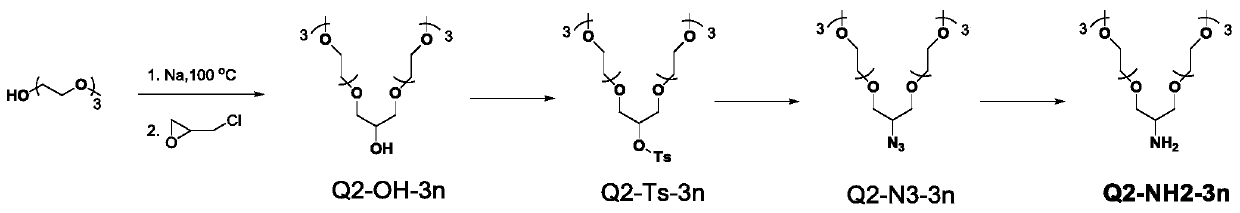
[0255] see figure 1 , figure 1 It is the synthetic route diagram of the monomer Q2-NH2-3n provided by Example 1 of the present invention.
[0256] according to figure 1 The shown route synthesizes monomer Q2-NH2-3n, and the specific process includes:
[0257] Triethylene glycol methyl ether (25 g, 0.16 mol) was heated to 100°C under the protection of argon, sodium wire (1.2 g, 0.055 mol) was slowly added, and after reacting at 100°C for 1 h, chloromethyl epoxy was slowly added Ethane (4.63 g, 0.055 mol), continued stirring at this temperature, and ended the reaction after 6 hours; filtered to remove solid impurities after cooling down, and distilled under reduced pressure to obtain a colorless and transparent Q2-OH-3n monomer.
[0258]The monomer Q2-OH-3n (14.57 grams, 0.038 moles) and sodium hydroxide (2.27 grams,) were dissolved in the mixed solution of tetrahydrofuran and water (volume ratio 100mL / 150mL), ...
Embodiment 2
[0281] see Figure 9 , Figure 9 It is a synthetic route diagram of the polymer P-1 provided in Example 2 of the present invention.
[0282] according to Figure 9 The shown route preparation repeating unit is the conjugated polymer P1 of I-1 formula structure (the polymer obtained by method one is PI-1a, the polymer obtained by method two is PI-1b), and the specific process includes:
[0283] Method 1: Monomer III-2-Br3n (88.2 mg), monomer dithiophene (15.9 mg), tris(dibenzylideneacetone)dipalladium Pd 2 (dba) 3 (0.19 mg), PivOH trimethylacetate (0.22 mg), and potassium carbonate (0.52 mg) were placed in a dry microwave vial, the vial was sealed with a septum cap, evacuated, and filled with argon three times, and then added dry anaerobic ophthalmic Xylene (o-xylene, 0.8 ml), and then the mixture was reacted in a microwave reactor (210°C, 300w) for 20 minutes. After the reaction was finished, it was cooled to room temperature, the reaction mixture was diluted with chlorob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com