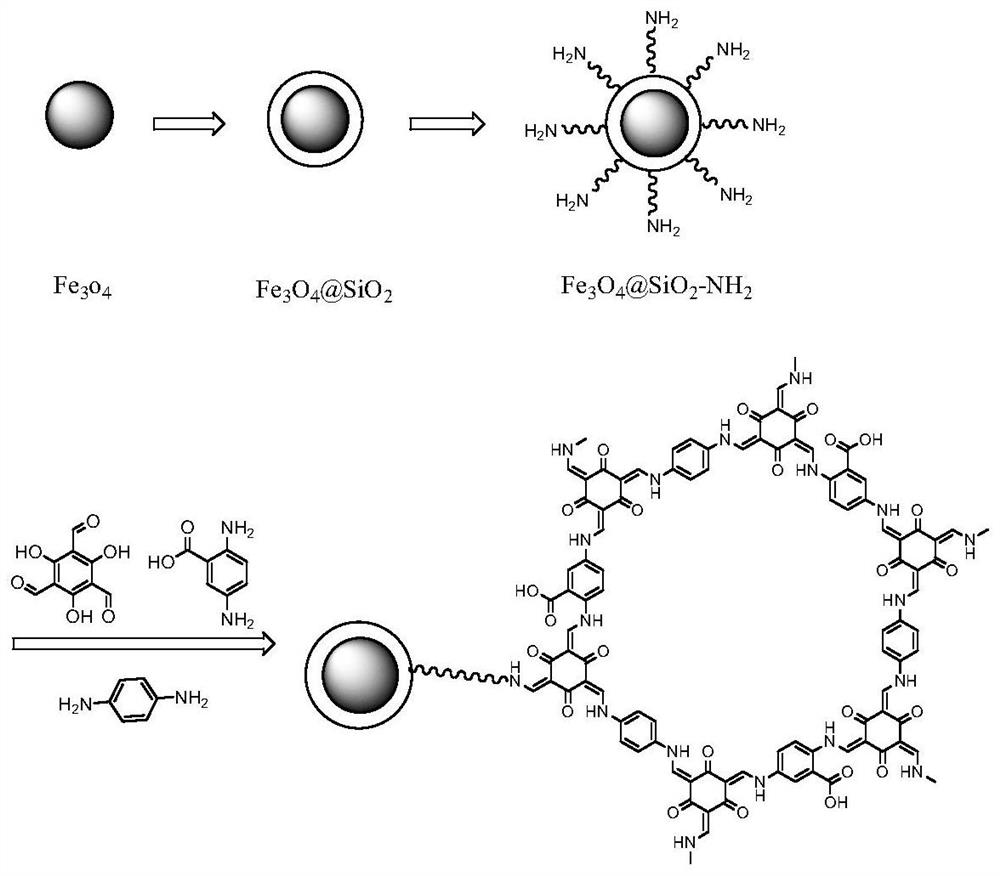
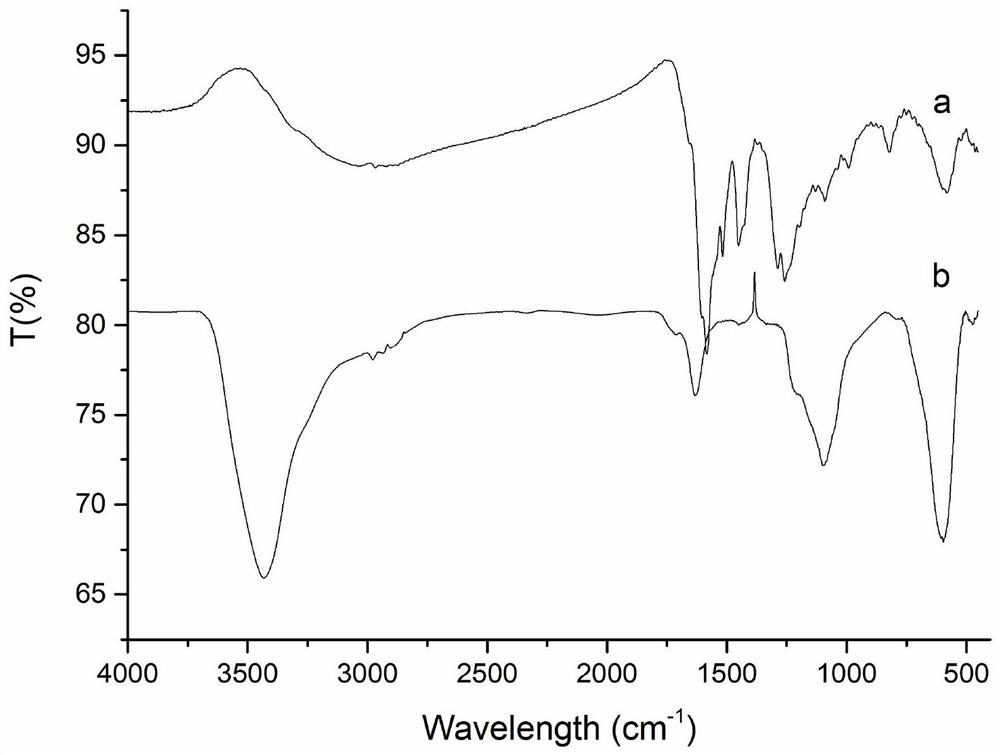
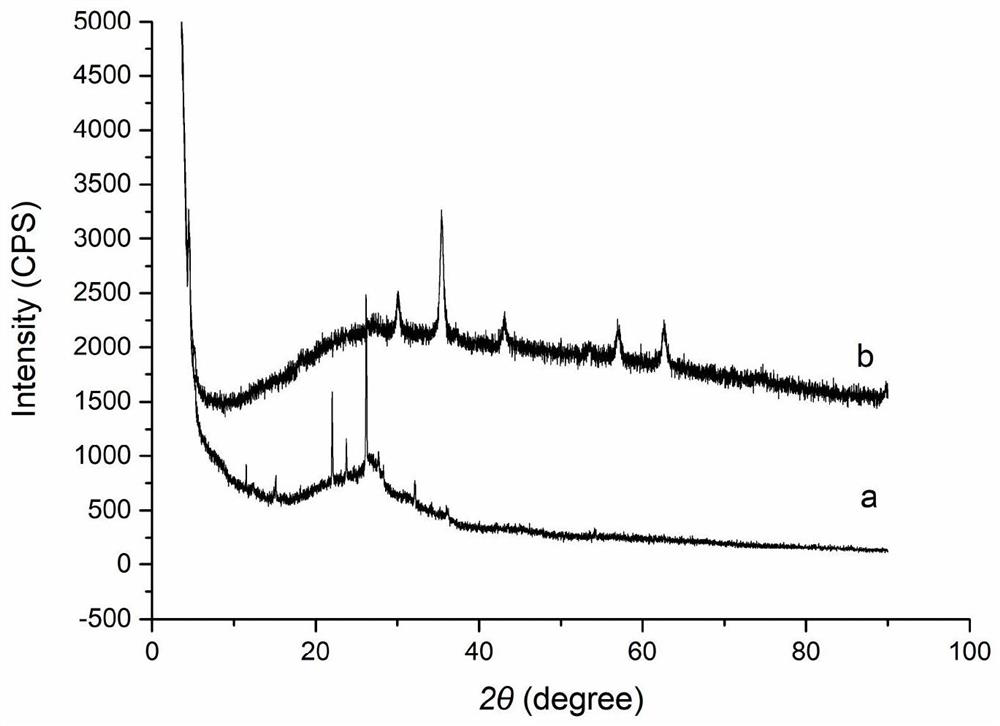
Magnetic carboxylated covalent organic framework nanocomposite material and its preparation method and application
A technology of covalent organic framework and nanocomposite materials, applied in chemical instruments and methods, alkali metal compounds, inorganic chemistry, etc., can solve the problems of large solution consumption, time-consuming, single action mode of the extraction target, etc., and achieve pore Large, improved adsorption efficiency, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. Preparation of magnetic carboxylated covalent organic framework nanocomposites
[0043] A method for preparing a magnetic carboxylated covalent organic framework nanocomposite material, comprising the following steps:
[0044] (1) Magnetic Fe 3 o 4 Synthesis of nanoparticles:
[0045] Weigh 2.7g FeCl 3 ·6H 2 O solid in a 100mL beaker, add 80mL of ethylene glycol, ultrasonic to FeCl 3 ·6H 2 O solid dissolved solution is a transparent liquid, then add 7.2g of anhydrous sodium acetate and 2.0g of polyethylene glycol, ultrasonic 20min, the solution is yellowish brown, and contains a large amount of yellow flocs; the resulting mixture is transferred to the autoclave , reacted at 200 ° C for 12 h, cooled to room temperature, and washed the Fe produced by the reaction with ethanol and double distilled water in sequence. 3 o 4 , repeated three times, stored in absolute ethanol for later use.
[0046] (2) Magnetic Fe 3 o 4 @SiO 2 Synthesis of nanoparticles...
Embodiment 2
[0058] Example 2. Adsorption performance test of magnetic carboxylated covalent organic framework nanocomposites for polycyclic aromatic hydrocarbons
[0059] Weigh 4 mg magnetic carboxylated covalent organic framework nanocomposites, add 0.2 μg / mL polycyclic aromatic hydrocarbons mixed standard (naphthalene, fluorene, anthracene, phenanthrene, pyrene, )5mL, ultrasonically adsorbed for 2min, 3min, 4min, 5min, 7min, 9min, and 11min respectively, and the supernatant was filtered through a 0.2μm water filter membrane, and then injected for analysis to investigate the effect of adsorption time on the adsorption efficiency of polycyclic aromatic hydrocarbons. from Figure 5 It can be seen that with the increase of adsorption time, the adsorption efficiency gradually increases, and when the adsorption time is 5 min, the adsorption efficiency can reach more than 95%.
Embodiment 3
[0060] Example 3, Test of Adsorption Performance of Magnetic Covalent Organic Framework Composite Materials on Tetracycline
[0061] Weigh 4mg of magnetic carboxylated covalent organic framework nanocomposite, add 0.2μg / mL tetracycline mixed standard (oxytetracycline, tetracycline, methacycline, doxycycline) 5mL, ultrasonic adsorption for 2min, 4min, 6min respectively , 8min, 10min, 12min, 15min, the supernatant was filtered through a 0.2μm water filter membrane, and the sample was analyzed to investigate the influence of the adsorption time on the tetracycline adsorption efficiency. from Image 6 It can be seen that with the increase of adsorption time, the adsorption efficiency gradually increases, and when the adsorption time is 8 min, the adsorption efficiency can reach more than 93.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com