Wide-band-gap copolymer acceptor material based on perylene diimide and preparation method thereof
A technology of perylene diimide and acceptor materials, which is applied in the field of wide bandgap copolymer acceptor materials and its preparation, can solve the problems of high molar extinction coefficient, narrow light absorption range, high short-circuit current function, etc., and achieve high molar extinction coefficient, widening spectrum, effect of high short-circuit current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The perylene diimide-porphyrin-3,3'-difluoro-2,2'bithiophene random copolymer acceptor with a porphyrin content of 10% provided by the embodiment of the present invention has the following synthesis route:
[0052]
[0053] Synthesis of Compound 2: Dissolve Compound 1 (3.002g, 3.98mmol) in a 250mL two-necked flask with 10mL of dichloromethane, add Br 2 (10ml, 195.17mmol), stirred at room temperature for 3 days. Slowly introduce the solution into excess saturated sodium thiosulfate solution under ice bath, stir for one hour, then wash the mixture several times with deionized water, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, spin dry the solvent, Using petroleum ether:dichloromethane (v:v=4:1) as the developing solvent, the red crystal 2 (762 mg, 21%) was obtained by column separation. 1 H NMR (400MHz, Chloroform-d) δ9.49 (d, J = 8.2Hz, 2H), 9.04–8.85 (m, 2H), 8.78–8.52 (m, 2H), 5.30–5.03 (m, 2H), 2.23 (m, 4H), 1.84 (m, 4H), 1.49–1.06 (m...
Embodiment 2
[0061] The perylene diimide-porphyrin-3,3'-difluoro-2,2'bithiophene random copolymer acceptor with a porphyrin content of 5% provided by the embodiment of the present invention has the following synthesis route:
[0062]
[0063] Synthesis of copolymer P2: under argon protection, add compound 2 (135.2 mg, 0.15 mmol), compound 7 (8.3 mg, 0.0078 mmol), (3,3'-difluoro-[2, 2'-bithiophene]-5,5'-diyl)bistrimethyltin (82.3mg, 0.16mmol), tetrakis(triphenylphosphine)palladium (9mg, 0.0078mmol), 0.3mL DMF and 1.2mL toluene , stirred at 110°C for 24 hours. The product precipitated at the bottom of the bottle, and the reaction was stopped. The reaction solution was cooled to room temperature, sucked up with a dropper and added dropwise to the methanol solution. At this time, the solid crude product precipitated, filtered, and dried in vacuo. Through a Soxhlet extractor, extract with acetone and petroleum ether for one day to remove small molecules and other by-products, and then extra...
Embodiment 3
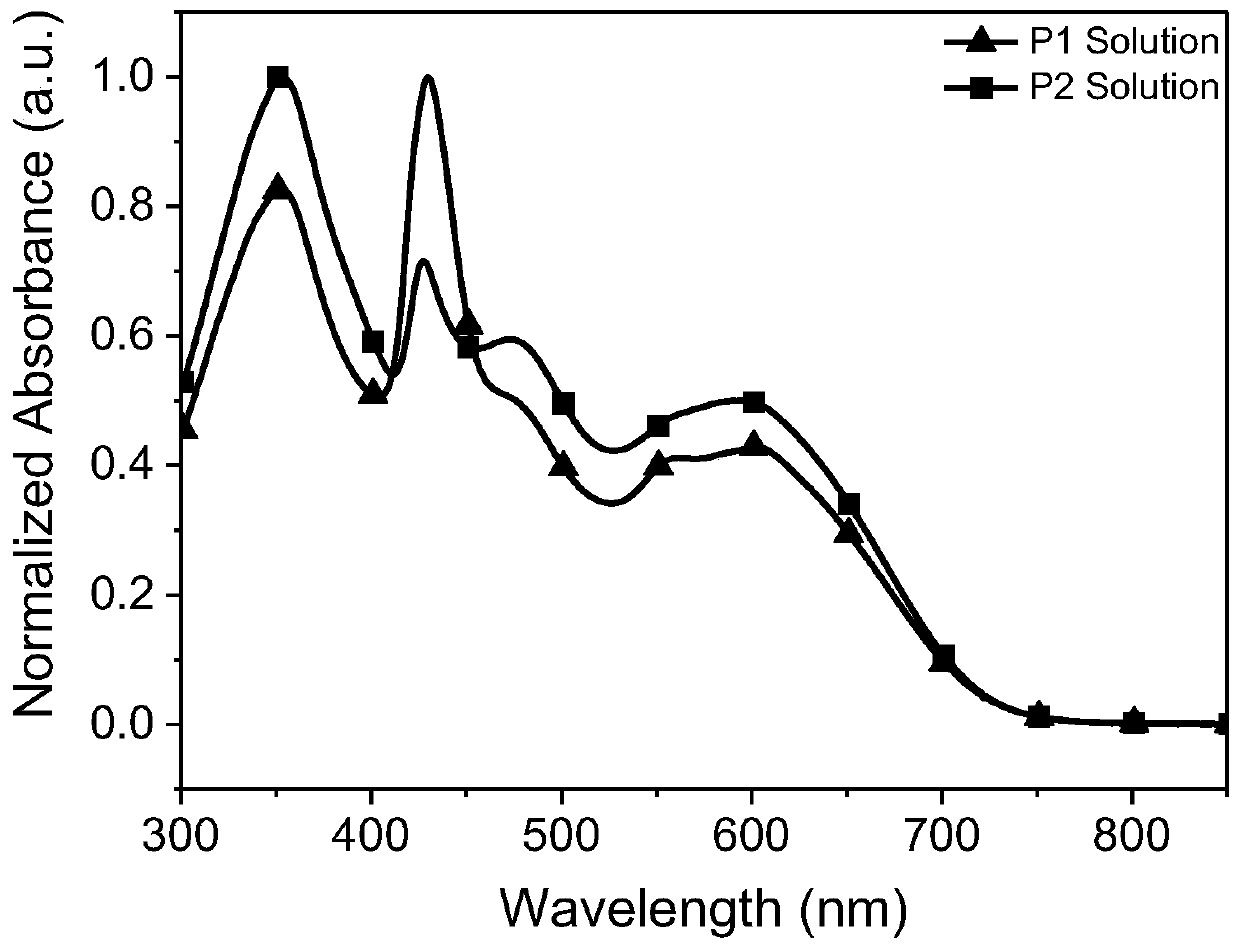
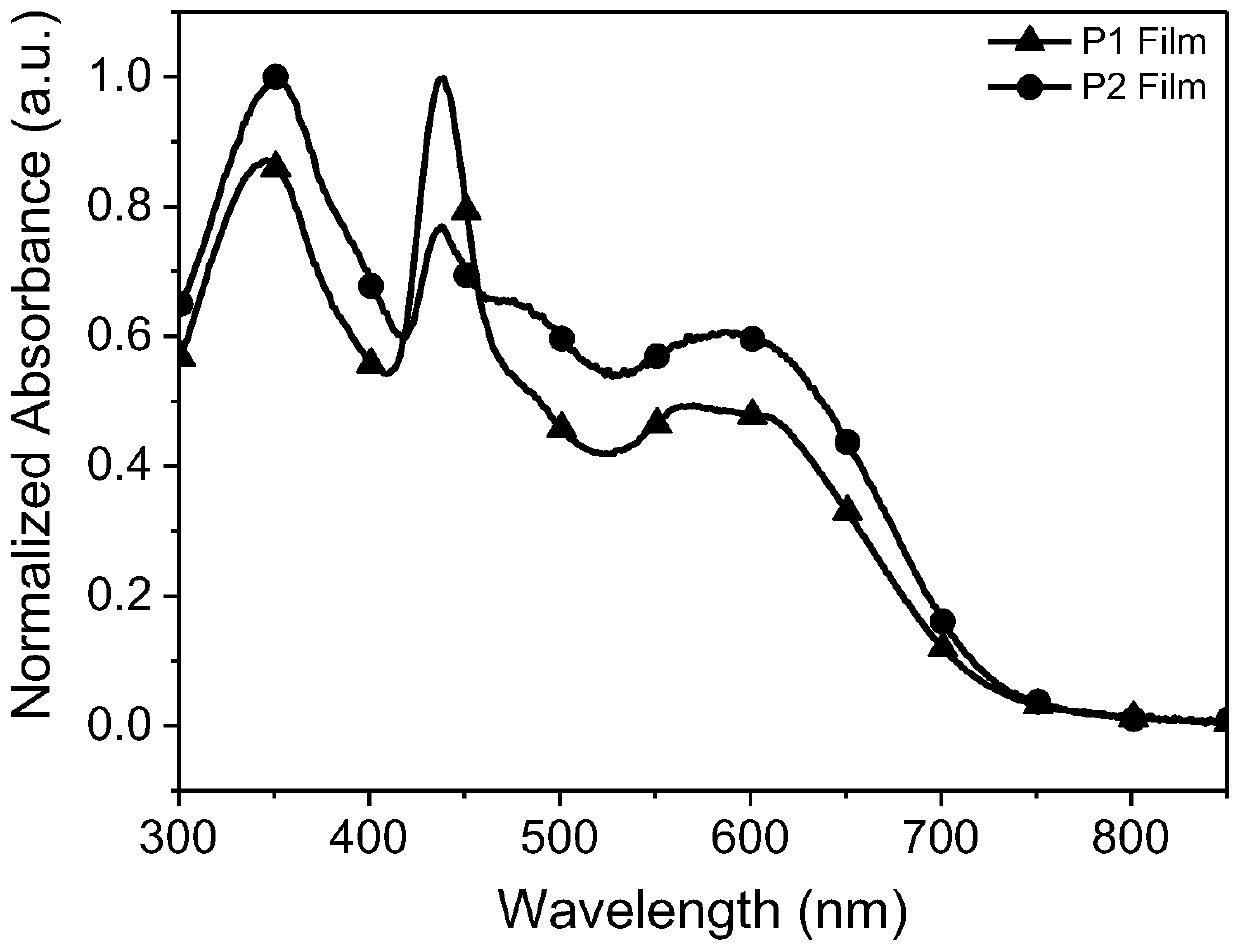
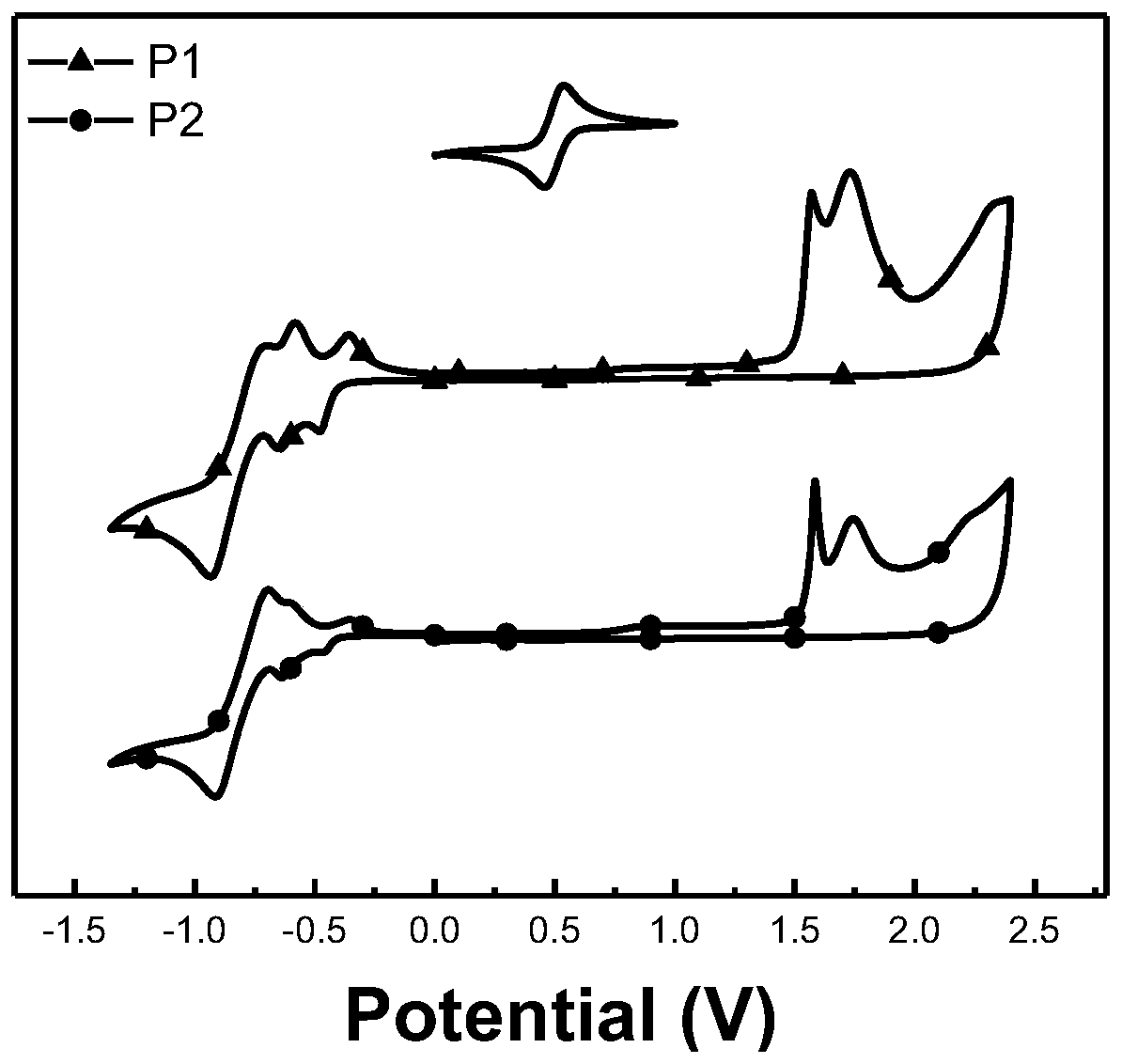
[0065] The absorption spectra of acceptors P1 and P2 of the perylenediimide-porphyrin-3,3'-difluoro-2,2'bithiophene random copolymer provided in the examples of the present invention are as follows.
[0066] figure 1 and figure 2 UV-Vis absorption spectra of perylenediimide-porphyrin-3,3’-difluoro-2,2’-bithiophene random copolymer acceptors P1 and P2 in chloroform solution and on quartz plate, respectively.
[0067] Depend on figure 2It can be seen that the maximum values of film absorption of P1 and P2 are respectively at about 346, 439, 566nm and 352, 439, 590nm, and the peak values are respectively at about 719nm and 722nm, and the optical band gap is 1.72eV (the optical band gap can be calculated according to the formula Eg= 1240 / λonset calculation, where Eg is the optical bandgap, which is the maximum absorption sideband value absorbed by the λonset film), and the data are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com