Stem cell injection for treating osteoarthritis cartilage defect and preparation and application thereof
A stem cell technology for osteoarthritis, applied in the field of stem cell injection and its preparation for the treatment of cartilage defects in osteoarthritis, to achieve the effect of large number of cells, promotion of damage repair, and good microbiological and biological safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: The above-mentioned cultured human umbilical cord mesenchymal stem cells are used to prepare stem cell injection for treating arthritis. The specific preparation process is as follows:
[0080] Dispense the washing solution (DPBS) into 15ml centrifuge tubes for later use; turn on the centrifuge and adjust to 1900rpm for 6min; prepare the liquid medium: autologous platelet-rich plasma, human albumin, cell strainer, normal saline; Take out the corresponding number of cryopreserved cells from the nitrogen tank, and quickly transfer them to a 37°C water bath; when the ice cubes in the cell cryopreservation tubes are as large as soybeans (water bath time 1.5-2.5 minutes), take out the cryopreservation tubes, and quickly transfer them to the preparation Area. Wipe the cryovial with sterile alcohol for disinfection, and unscrew the cap; transfer the cells to the prepared washing solution with a pipette gun, centrifuge at 1900rpm for 6min. After centrifugation, remo...
Embodiment 2
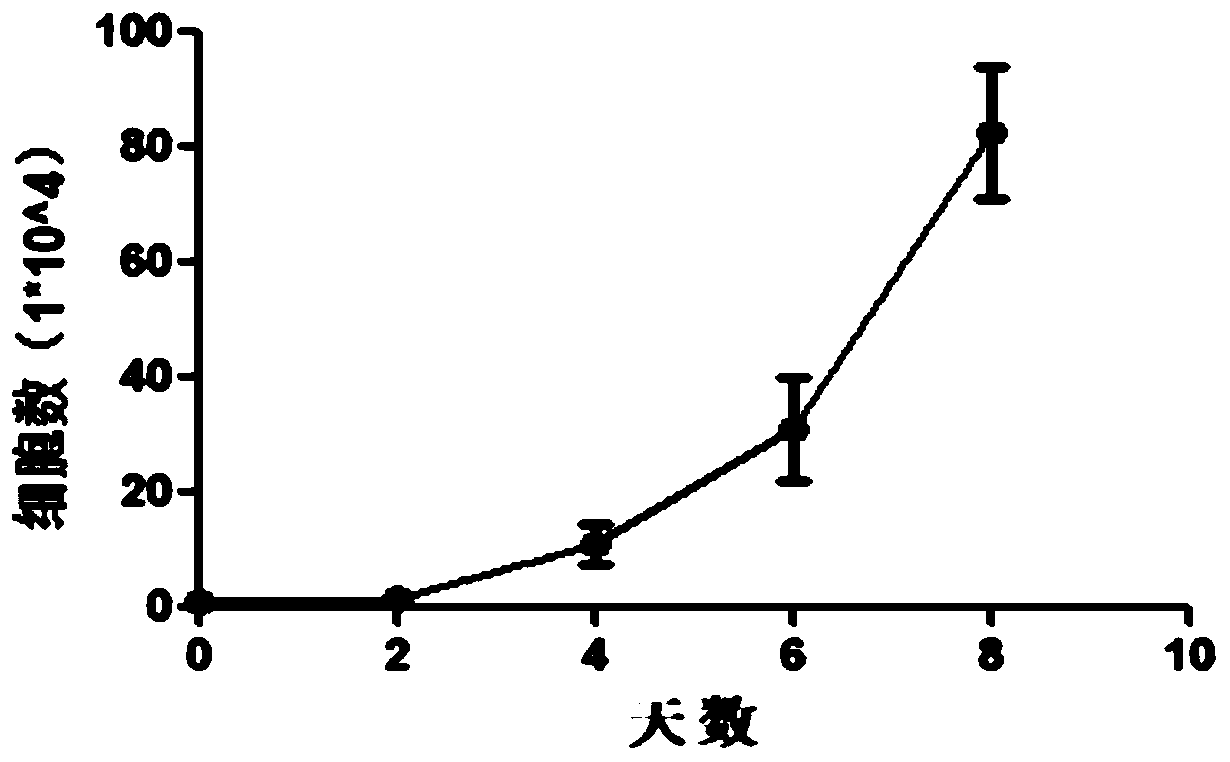
[0086] Example 2: Study on the curative effect of P5 generation UC-MSCs on knee osteoarthritis animal model In this experiment, the cell therapy composition 1 (stem cells + normal saline) prepared in Example 1 was selected to investigate its effect on knee osteoarthritis Curative effect, its specific experimental process is as follows:
[0087] Select 60 adult healthy male Wistar rats (weight 175g~200g) and divide them into four groups according to random number table method: normal group (n=15), model group (n=15), stem cell treatment group (n=15), HA treatment group (n=15). The normal group was rats without any treatment, the model group was rats with knee osteoarthritis induced by sodium iodoacetate (Monosodium iodoacetate, MIA), the stem cell treatment group was rats injected with UC-MSCs into the knee joint cavity, and the rats treated with HA In the group, HA was injected into the knee joint cavity. After modeling, 14 days after knee osteoarthritis. The stem cell ther...
Embodiment 3
[0090] Example 3: Clinical application of the composition 3 (stem cells + autologous platelet-rich plasma) prepared in Example 1 to knee osteoarthritis Case 1:
[0091] Basic patient information: Patient Zhao XX, male, 51 years old, occupation: policeman / martial arts athlete. Physical examination in November 2018, chief complaint: repeated knee joint pain for 20+ years, aggravated for 4 years; current medical history: patient's double knee joint pain was gradually aggravated due to long-term heavy exercise, and both knee joint pains were felt when walking and resting , especially on the right. The movement of the right knee joint was accompanied by popping sound. Acupuncture, local injection of sodium hyaluronate and other treatments had no obvious effect. The pain gradually increased in the past 4 years, accompanied by deformity of the right knee joint. Since the onset, the spirit, food and drink are normal, and the sleep is not good enough. Past history: In 2014, the right...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com