A kind of in-situ preparation method and application of polymer electrolyte
An in-situ preparation and polymer technology, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolytes, solid electrolytes, etc., can solve the problems of reducing the decomposition of liquid electrolytes, shorten the preparation cycle, simplify the preparation process, and solve the problem of leakage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The in-situ preparation method of a polymer electrolyte provided in this embodiment is as follows:
[0030] S1: Under the conditions of anhydrous and isolated oxygen, the monomer methyl methacrylate and the polyethylene glycol methyl ether methacrylate with a relative molecular mass of 300, the initiator 2-bromophenylacetate and lithium The reaction raw materials of salt lithium perchlorate are mixed, and the precursor liquid A1 is formed by stirring uniformly; the molar ratio of methyl methacrylate, polyethylene glycol methyl ether methacrylate, 2-bromophenyl ethyl acetate, and lithium perchlorate is 20:10:1:6.
[0031] The specific configuration process is: S1: Weigh 0.1425g of lithium perchlorate in the glove box and put it into the eggplant-shaped bottle, take 0.5ml of methyl methacrylate and 1ml of polyethylene glycol methyl ether methacrylate and add it to the eggplant-shaped bottle. In the bottle, after magnetic stirring for 20 minutes, 3 microliters of ethyl 2-...
Embodiment 2
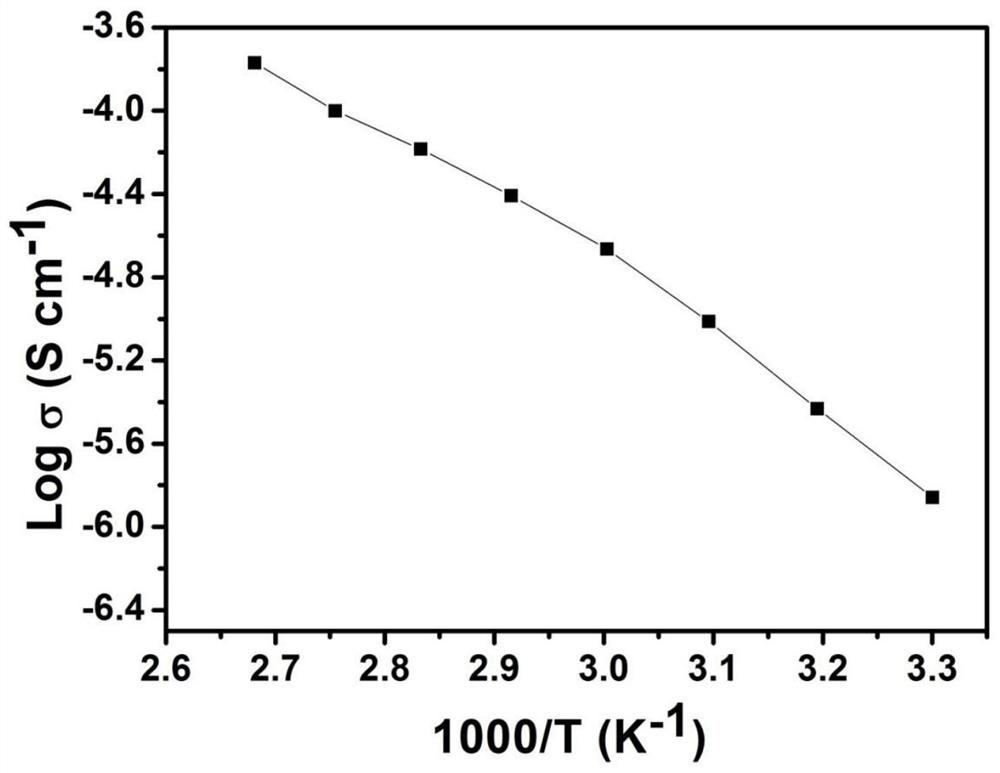
[0035] The difference between Example 2 and Example 1 is that the monomers are butyl methacrylate and polyethylene glycol methyl ether methacrylate with a relative molecular mass of 500, and the initiator is 2-iodo-2-methyl methacrylate. Propionitrile, the lithium salt is lithium hexafluorophosphate, stir evenly to form precursor solution B1, the molar ratio of butyl methacrylate, polyethylene glycol methyl ether methacrylate, 2-iodo-2 methyl propionitrile, and lithium hexafluorophosphate is 20:30: 1:8; the remaining steps are the same as in Example 1; the polymer B2 and the polymer electrolyte B3 with a number average relative molecular mass of 45300 are obtained, and the lithium ion conductivity of the polymer electrolyte B3 measured at room temperature is 7.8×10 -5 s / cm.
Embodiment 3
[0037] The difference between Example 3 and Example 1 is that the monomers are methyl acrylate, styrene and polyethylene glycol methyl ether methacrylate with a relative molecular mass of 475, and the initiator is 2-iodo-2-methyl. Propionitrile, the lithium salt is lithium dioxalate borate, stir well to form precursor liquid C1, methyl acrylate, styrene, polyethylene glycol methyl ether methacrylate, 2-iodo-2-methylpropionitrile, dioxalic acid The molar ratio of lithium borate is 20:10:20:1:9; the remaining steps are the same as in Example 1; the polymer C2 and the polymer electrolyte C3 with a number average relative molecular weight mass of 47300 are obtained, and the polymer electrolyte C3 is at room temperature. The measured lithium ion conductivity is 6.5×10 -5 s / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com