Proton-triggered nano-drug system with hydrophilic-hydrophobic-dimensional double conversion characteristics and preparation method and application thereof
A technology for converting properties and nano-drugs, which is applied in the field of nano-drug systems and its preparation, can solve problems such as limited tumor uptake rate and affinity, toxic side effects and drug resistance, and free radical scavenging activities that are easily affected by various factors. Achieve the effects of avoiding changes in pharmacodynamic behavior, reversing tumor multidrug resistance, and improving drug delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
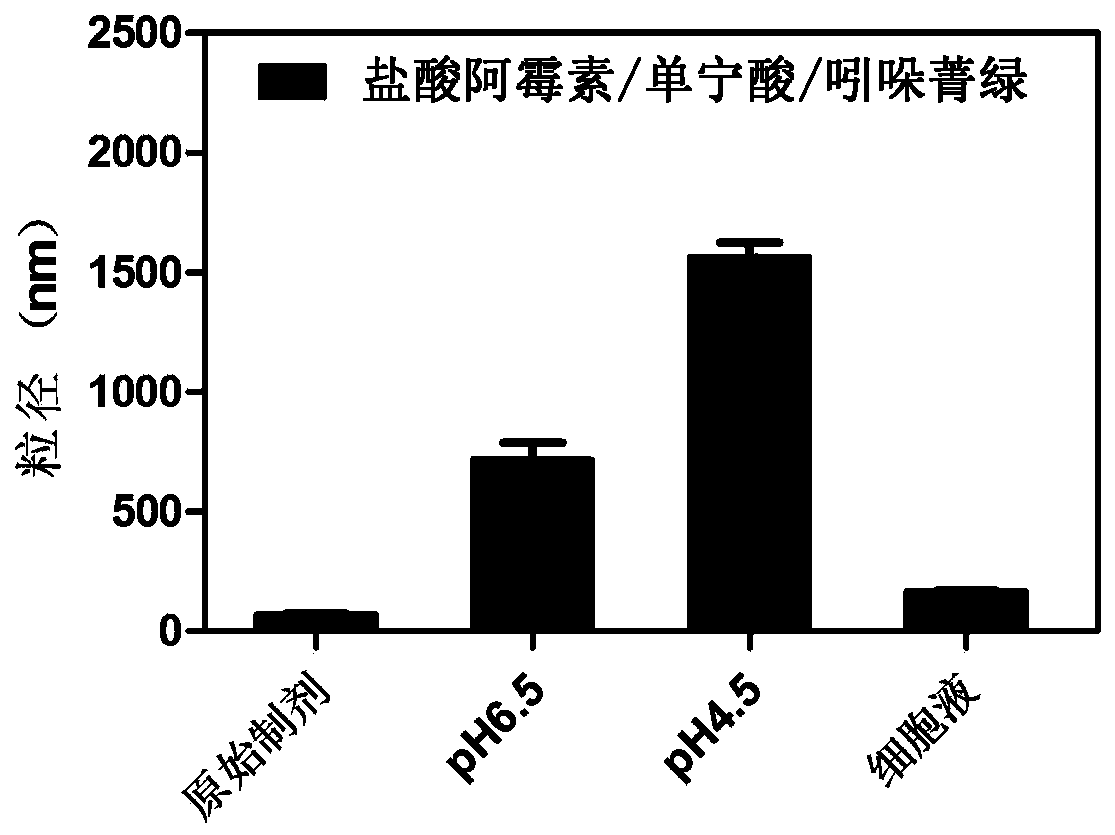
[0054] Embodiment 1: Preparation of doxorubicin hydrochloride / tannic acid / indocyanine green nano system
[0055] According to the mass ratio of 2:3:3, doxorubicin hydrochloride, tannic acid, and indocyanine green were precisely weighed and dissolved in ultrapure water, and vortexed to mix. Mix and stir the doxorubicin hydrochloride solution and the tannic acid solution, and inject ultrapure water whose volume is 10 times the sum of the volumes of the doxorubicin hydrochloride solution and the tannic acid solution under stirring conditions. After stirring for 5 minutes, inject the indocyanine green solution and continue stirring for 30 minutes. Centrifuge, take the precipitate, redissolve, and sonicate for 5 minutes to prepare a nanosystem based on proton-triggered hydrophilic-hydrophobic-size double conversion.
Embodiment 2
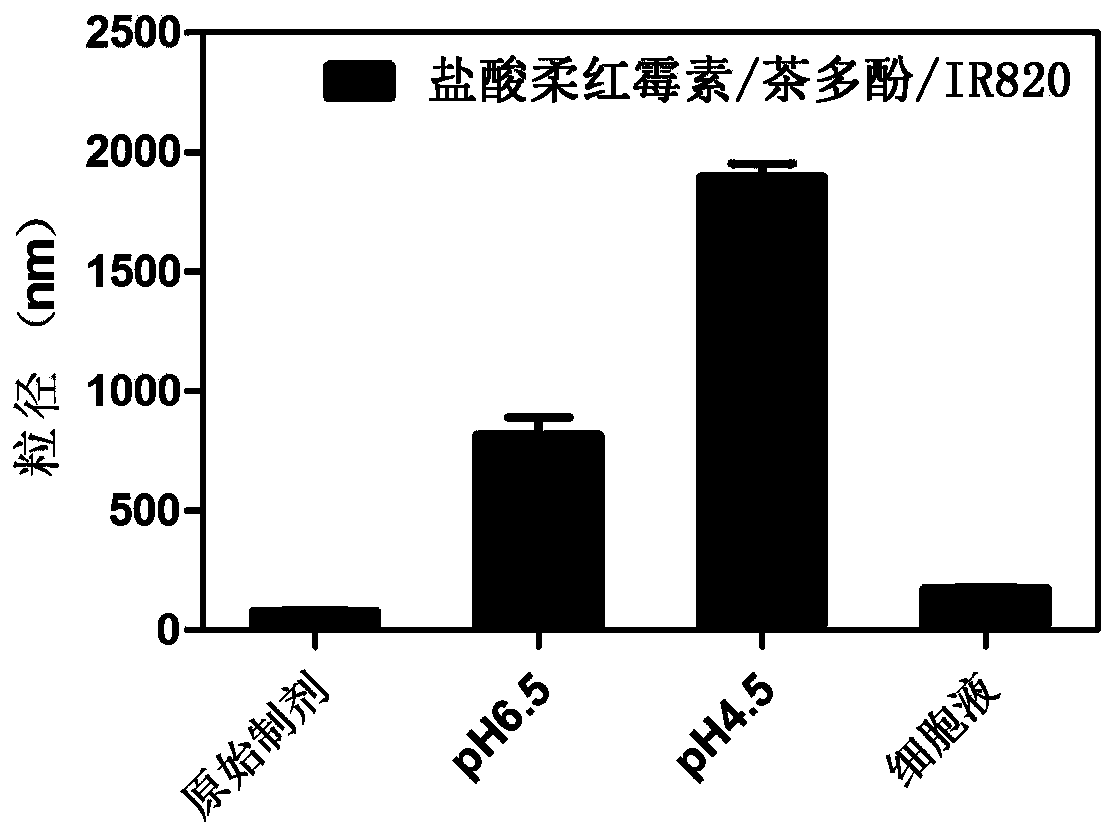
[0056] Embodiment 2: the preparation of daunorubicin hydrochloride / tea polyphenol / IR820 nanometer system
[0057] Accurately weigh daunorubicin hydrochloride, tea polyphenols, and IR820 in alcohol according to the mass ratio of 2:5:4, and vortex to mix. Mix and stir the daunorubicin hydrochloride solution and the tea polyphenol solution, and inject ultrapure water whose volume is 5 times the sum of the daunorubicin hydrochloride solution and the tea polyphenol solution under stirring conditions. After stirring for 10 minutes, inject IR820 aqueous solution and continue stirring for 40 minutes. Centrifuge, take the precipitate, redissolve, and sonicate for 10 minutes to prepare a nanosystem based on proton-triggered hydrophilic-hydrophobic-size double conversion.
Embodiment 3
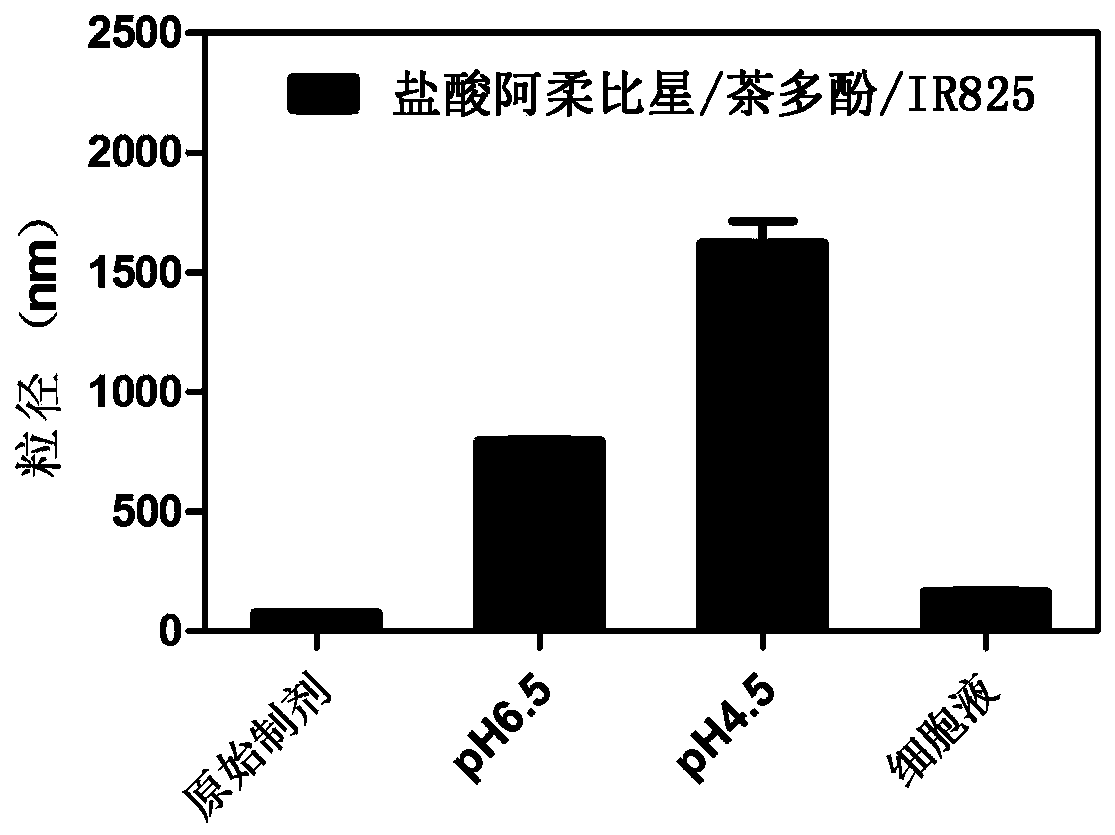
[0058] Embodiment 3: Preparation of arubicin hydrochloride / tea polyphenol / IR825 nanometer system
[0059]Accurately weigh arubicin hydrochloride, tea polyphenols, and IR825 according to the mass ratio of 4:10:8, dissolve them in ultrapure water, and vortex to mix. Mix and stir the arubicin hydrochloride solution and the tea polyphenol solution, and inject ultrapure water whose volume is 20 times the volume of the arubicin hydrochloride solution and the tea polyphenol solution under stirring conditions. After stirring for 8 minutes, inject IR825 aqueous solution and continue stirring for 20 minutes. Centrifuge, take the precipitate, redissolve, and sonicate for 15 minutes to prepare a nanosystem based on proton-triggered hydrophilic-hydrophobic-size double conversion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com