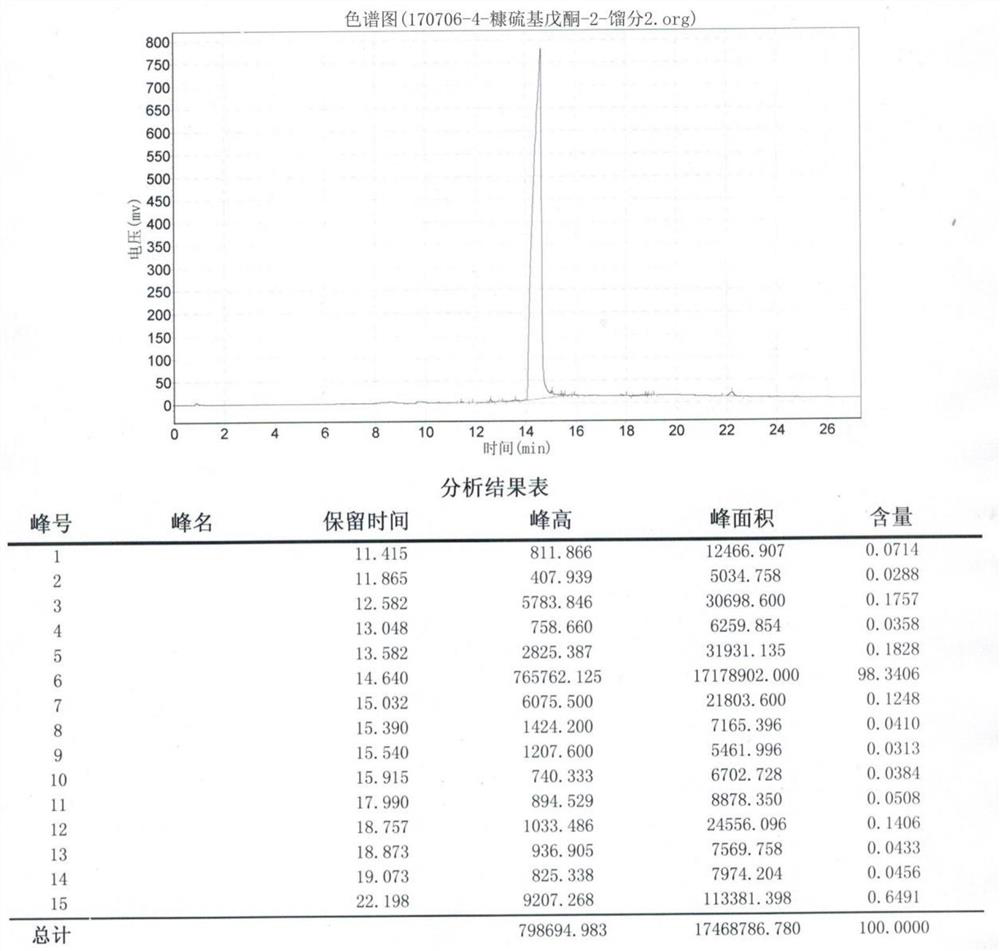
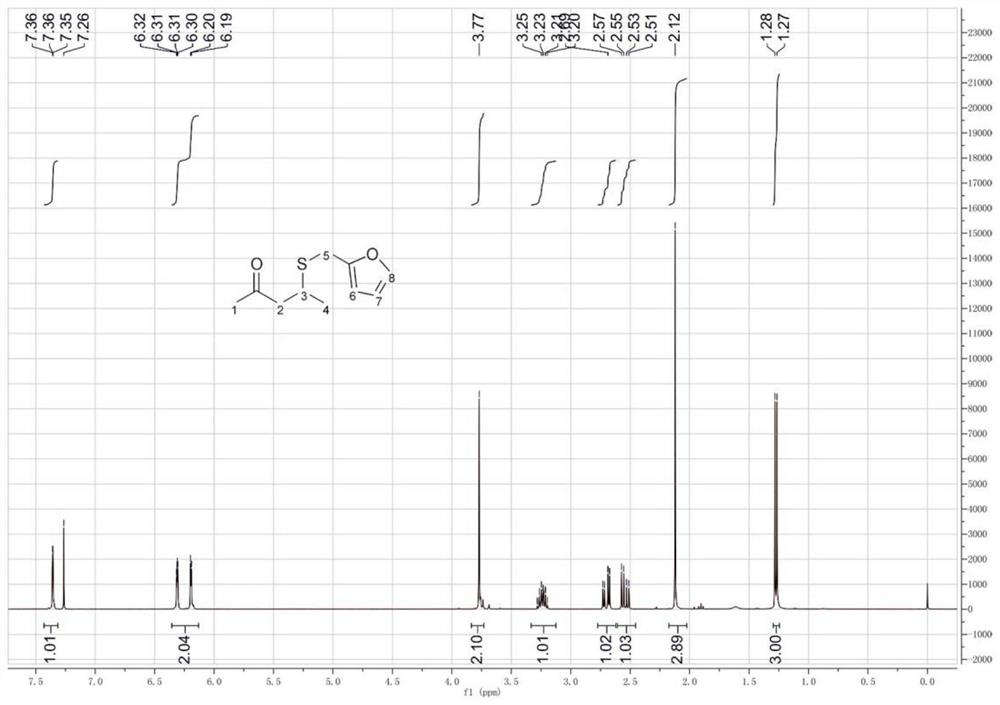
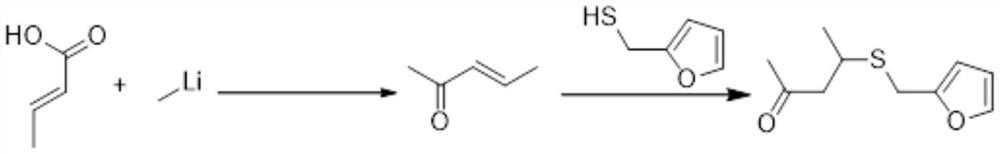
The preparation method of 4-furfurylthiopentanone-2
A technology of furfuryl thiopentanone and furfuryl mercaptan, which is applied in the field of fine chemistry, can solve the problems of high risk, high cost, unsuitability for industrial production and the like of the synthesis route, and achieves the optimization of the synthesis route, the improvement of the yield, and the guarantee of the product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of 4-furfurylthiopentanone-2 comprises the following steps:
[0041] S1. Add 15L of toluene to the reaction kettle, slowly add 9.4kg of triphenylphosphine, add 3kg of chloroacetone after it dissolves, start to heat up and reflux, and GC detects that the reaction is complete.
[0042] Post-processing: the reaction solution was cooled to room temperature and suction filtered, the filtrate was recovered with toluene and used mechanically, the filter cake was washed 3 times with 3L petroleum ether, and the solid was dried to obtain the phosphorus salt.
[0043] S2. Add the phosphorus salt obtained in step S1 to the reactor, add 10L of dichloromethane to dissolve, then cool 7.8kg of 30% sodium hydroxide aqueous solution to room temperature and add it to stir, and the reaction is completed in 2 to 3 hours;
[0044] Post-processing: liquid separation, the dichloromethane phase was washed with 5 L of saturated ammonium chloride until neutral, and washed w...
Embodiment 2
[0050] The preparation method of 4-furfurylthiopentanone-2 comprises the following steps:
[0051] S1. Add 15L of toluene to the reaction kettle, slowly add 9.4kg of triphenylphosphine, add 3kg of chloroacetone after it dissolves, start to heat up and reflux, and GC detects that the reaction is complete.
[0052] Post-processing: the reaction solution was cooled to room temperature and suction filtered, the filtrate was recovered with toluene and used mechanically, the filter cake was washed 3 times with 3L petroleum ether, and the solid was dried to obtain the phosphorus salt.
[0053] S2. Add the phosphorus salt obtained in step S1 to the reactor, add 10L of dichloromethane to dissolve, then cool 7.8kg of 30% sodium hydroxide aqueous solution to room temperature and add it to stir, and the reaction is completed in 2 to 3 hours;
[0054]Post-processing: liquid separation, the dichloromethane phase was washed with 5 L of saturated ammonium chloride until neutral, and washed wi...
Embodiment 3
[0060] The preparation method of 4-furfurylthiopentanone-2 comprises the following steps:
[0061] S1. Add 15L of toluene to the reaction kettle, slowly add 9.4kg of triphenylphosphine, add 3kg of chloroacetone after it dissolves, start to heat up and reflux, and GC detects that the reaction is complete.
[0062] Post-processing: the reaction solution was cooled to room temperature and suction filtered, the filtrate was recovered with toluene and used mechanically, the filter cake was washed 3 times with 3L petroleum ether, and the solid was dried to obtain the phosphorus salt.
[0063] S2. Add the phosphorus salt obtained in step S1 to the reaction kettle, add 10L tetrahydrofuran to dissolve it, cool the system to 0°C, add 1.7kg 60% sodium hydride in batches, and stir for 2 to 3 hours until the reaction is completed;
[0064] Post-processing: Concentrate to remove tetrahydrofuran, add 10L of dichloromethane and 2L of water to the residue, separate the liquids, wash the dichlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com