Method for removing pertussis component pilin 2/3 endotoxin
A technology of pili protein and whooping cough, applied in the field of biopharmaceuticals, can solve the problems of affecting the safety of vaccines, easy introduction of affinity groups, loss of target proteins, etc., and achieve the effect of maintaining biological activity and recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: method of the present invention
[0038] 1) Open a strain of Bacillus pertussis and inoculate it on the ginger medium, cultivate it at 36°C for 3 days, transfer it to the activated carbon agar medium, cultivate it at 36°C for 40 hours, and inoculate it in 5L pertussis liquid medium (MSS), The shake flask was cultivated for 20 hours, and as fermentor seeds, it was put into a 300L fermentor (working volume 150L) for cultivation, and harvested after cultivating for 34 hours. The harvested fermentation broth was separated from supernatant and pertussis bacteria using a continuous flow centrifuge.
[0039]2) After dissolving the pertussis cells obtained in step 1 with pH 7.0, 0.02 MPBS buffer solution containing 2M urea, stir at 2-8° C. for 30 min.
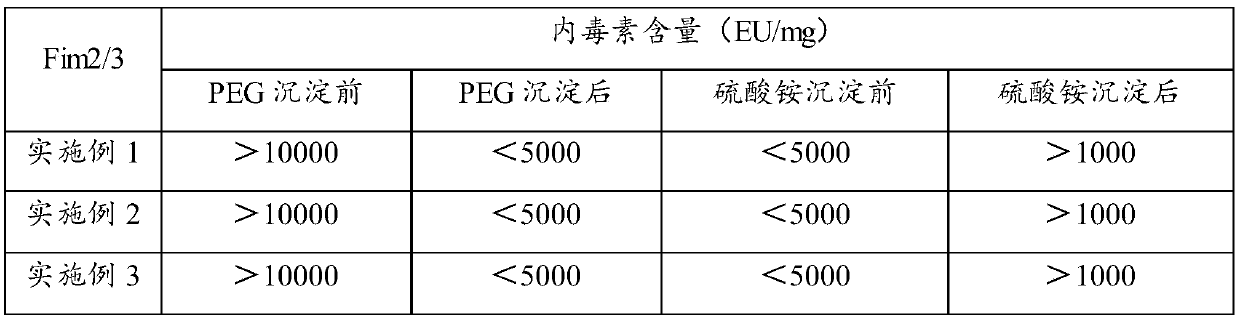
[0040] 3) Centrifuge (10000 rpm, 30 min) to collect the supernatant, heat at 80° C. for 30 min, and then centrifuge (10000 rpm, 30 min) to collect the supernatant. PEG8000 was added to the clarified supernatant t...
Embodiment 2
[0044] Embodiment 2: method of the present invention
[0045] 1) Open a strain of Bacillus pertussis and inoculate it on the ginger medium, culture it at 36.5°C for 3 days, transfer it to the activated carbon agar medium, cultivate it at 36.5°C for 42 hours, and inoculate it in 5L pertussis liquid medium (MSS), Cultivate in shake flasks for 20-24 hours, put them into a 300L fermenter (working volume: 150L) as fermenter seeds, and cultivate them for 35 hours before harvesting. The harvested fermentation broth was separated from supernatant and pertussis bacteria using a continuous flow centrifuge.
[0046] 2) After dissolving the pertussis cells obtained in step 1 with pH 7.2, 0.02 MPBS buffer solution containing 4M urea, stir at 2-8° C. for 30 min.
[0047] 3) Centrifuge (10000 rpm, 30 min) to collect the supernatant, heat at 80° C. for 30 min, and then centrifuge (10000 rpm, 30 min) to collect the supernatant. PEG8000 was added to the clarified supernatant to a final concen...
Embodiment 3
[0051] Embodiment 3: method of the present invention
[0052] 1) Open a strain of Bacillus pertussis and inoculate it in ginger medium, culture it at 37°C for 3 days, transfer it to activated carbon agar medium and culture it at 37°C for 43 hours, inoculate it in 5L pertussis liquid medium (MSS), shake The bottle was cultivated for 23 hours, and used as fermenter seeds, put into a 300L fermenter (working volume 150L) for cultivation, and harvested after cultivating for 35 hours. The harvested fermentation broth was separated from supernatant and pertussis bacteria using a continuous flow centrifuge.
[0053] 2) After dissolving the pertussis cells obtained in step 1 with pH 7.4 and 0.02M PBS buffer containing 6M urea, stir at 2-8°C for 30 min.
[0054] 3) Centrifuge (10000 rpm, 30 min) to collect the supernatant, heat at 80° C. for 30 min, and then centrifuge (10000 rpm, 30 min) to collect the supernatant. Add PEG8000 to the clarified supernatant to a final concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com