A compound containing α-substituted phenyl structure, its preparation method and disinfectant
A compound, phenyl technology, applied in the field of disinfectants, compounds containing α-substituted phenyl structures and their preparation, can solve the problems of poor bactericidal effect and large taste, and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
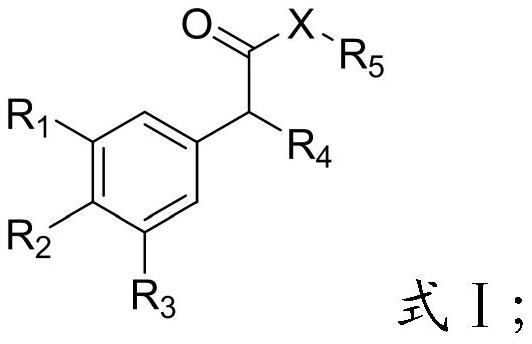
[0034] The present invention provides the preparation method of the compound containing α-substituted phenyl structure described in the technical scheme, when R 5 When -X- is a hydroxyl group, the preparation method of the compound containing α-substituted phenyl structure comprises the following steps:
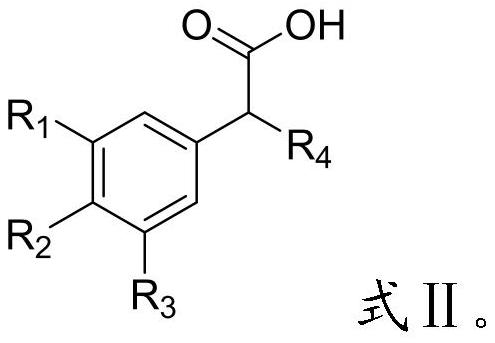
[0035] Will R 4 -H, glyoxylic acid and catalyst I are mixed, Friedel-Crafts reaction occurs, and a compound having a structure shown in formula I is obtained;
[0036] When R 5 When -X- is other groups except hydroxyl, the preparation method of the compound containing α-substituted phenyl structure comprises the following steps: R 4 -H, glyoxylic acid and catalyst I are mixed, and a Friedel-Crafts reaction occurs to obtain a compound of the structure shown in formula II;
[0037] The compound of the structure shown in the formula II, R 5 -X-H is mixed with catalyst II to undergo a condensation reaction to obtain a compound with the structure shown in formula I;
[00...
Embodiment 1
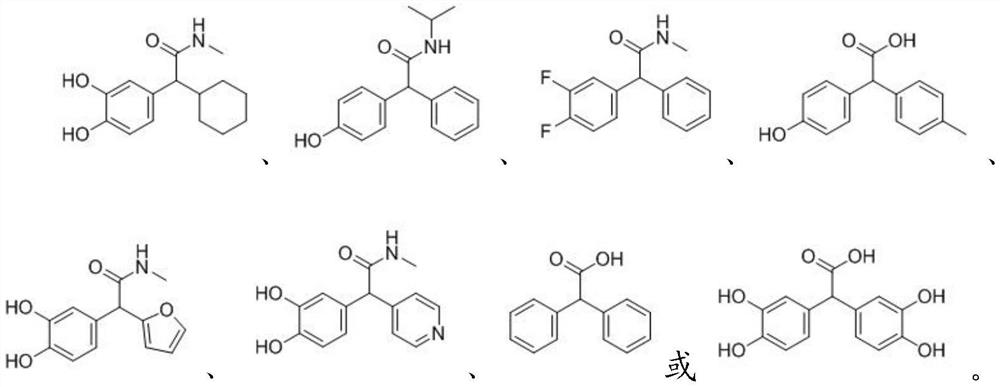
[0063] Add 500mg of catechol, 420mg of cyclohexane and 0.485mL of glyoxylic acid monohydrate into the reaction flask in sequence; use 10mL of water as a solvent, in the presence of 769mg of p-toluenesulfonic acid catalyst, under the condition of 80℃, at 160r Stir at a stirring speed of 1 / min for 5 hours, and TLC detects the reaction; after the reaction is complete, after cooling to room temperature, adjust the pH value of the reaction solution to 2, extract with ethyl acetate to obtain the crude product 2-cyclohexyl-2-(3,4-dihydroxy Phenyl) acetic acid; Then use ethanol recrystallization to obtain 1g of 2-cyclohexyl-2-(3,4-dihydroxyphenyl) acetic acid;
[0064] Dissolve the above-mentioned 2-cyclohexyl-2-(3,4-dihydroxyphenyl)acetic acid in 10mL of DMF, add 1.8gHATU, 3.3ml DIPEA and 1mL of 40% methylamine aqueous solution in sequence respectively, at 25°C , stirred at a stirring speed of 180r / min for 2h, TLC detected the reaction, and after the reaction was completed, use colum...
Embodiment 2
[0068] Add 500mg of 4-hydroxybenzene, 456mg of benzene and 0.568mL of glyoxylic acid monohydrate into the reaction flask in sequence; use 10mL of water as solvent, in the presence of 300mg of solid strong acid catalyst, at 70°C, at 160r / min Stir at a stirring speed for 5 hours, and detect the reaction by TLC; after the reaction is complete, after cooling to room temperature, adjust the pH value of the reaction solution to 2, and extract with ethyl acetate to obtain the crude product 2-(4-hydroxyphenyl)-N-isopropyl-2 -phenylacetic acid; then recrystallized with ethanol to obtain 1.1g of 2-(4-hydroxyphenyl)-N-isopropyl-2-phenylacetic acid;
[0069] The above-mentioned 2-(4-hydroxyphenyl)-N-isopropyl-2-phenylacetic acid was dissolved in 20mL of DMF, and 2.2gHATU, 3.9DIPEA and 1.1mL of isopropylamine were added successively. The stirring speed of min was stirred for 2h, and the reaction was detected by TLC. After the reaction was completed, it was purified by column chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com