Phosphodiesterase-4 inhibitor pharmaceutical composition for treatment of oral ulcer and preparation method thereof
A technology for phosphodiesterase and oral ulcers, which is applied in the direction of drug combination, drug delivery, and pharmaceutical formulations. Multiple administrations, stable and reliable quality, and fast onset of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
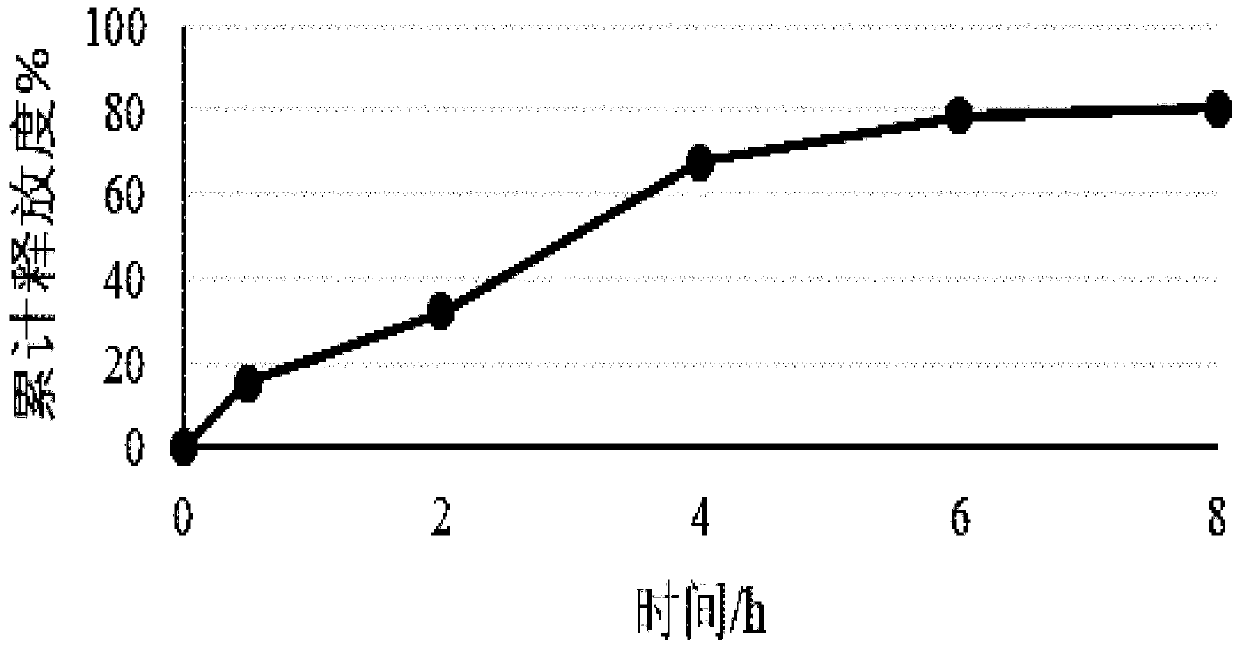
Image
Examples
Embodiment 1
[0027] Table 1 Composition of lyotropic liquid crystal drug carrier 1
[0028] prescription composition %(w / w) Apster 0.5 Glyceryl Dioleate 15 Lecithin 30 ethanol 15 Propylene Glycol 15 Tween 80 20 Menthic Acid 4.5
[0029] Preparation Process:
[0030] a) Add glyceryl dioleate into a stainless steel container, add propylene glycol and stir continuously with a spiral stirrer, mix well, and set aside;
[0031] b) Slowly add the bulk drug of Apremilast into ethanol, and add Tween 80 to fully dissolve the bulk drug, then add citric acid while stirring, mix well, and set aside;
[0032] c) adding the dissolved raw material drug into the lecithin, after stirring evenly, slowly adding the mixture of glyceryl dioleate and propylene glycol, and stirring evenly;
[0033] d) Put the above mixture in a water bath, the temperature of the water bath is 40-60°C, and the time of the water bath is 0.5-1h;
[0034] e) The mixture aft...
Embodiment 2
[0036] Table 2 Lyotropic liquid crystal drug carrier composition 2
[0037] prescription composition %(w / w) Apster 3 ethyl oleate 20 Lecithin 40 DMSO 6 glycerin 20 distilled water 5 citric acid 6
[0038] Preparation Process:
[0039] a) Ethyl oleate is added to a stainless steel container, glycerin is added and stirred continuously with a spiral agitator, mixed evenly, and set aside;
[0040] b) Dissolving the Apremilast bulk drug in DMSO, fully dissolving;
[0041] c) adding the dissolved raw drug into lecithin, stirring evenly, slowly adding a mixture of ethyl oleate and glycerin, placing it in a water bath at 50-70°C, then adding distilled water to the above mixture, fully stirring and mixing uniformly;
[0042]d) The above mixed mixture is centrifuged for 10-20 min to remove air bubbles, and then placed at 25-37° C. for a week to obtain a stable lyotropic liquid crystal drug carrier.
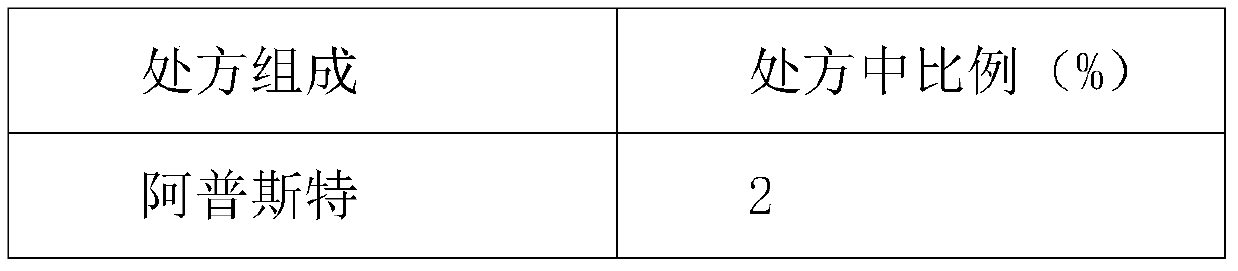
Embodiment 3
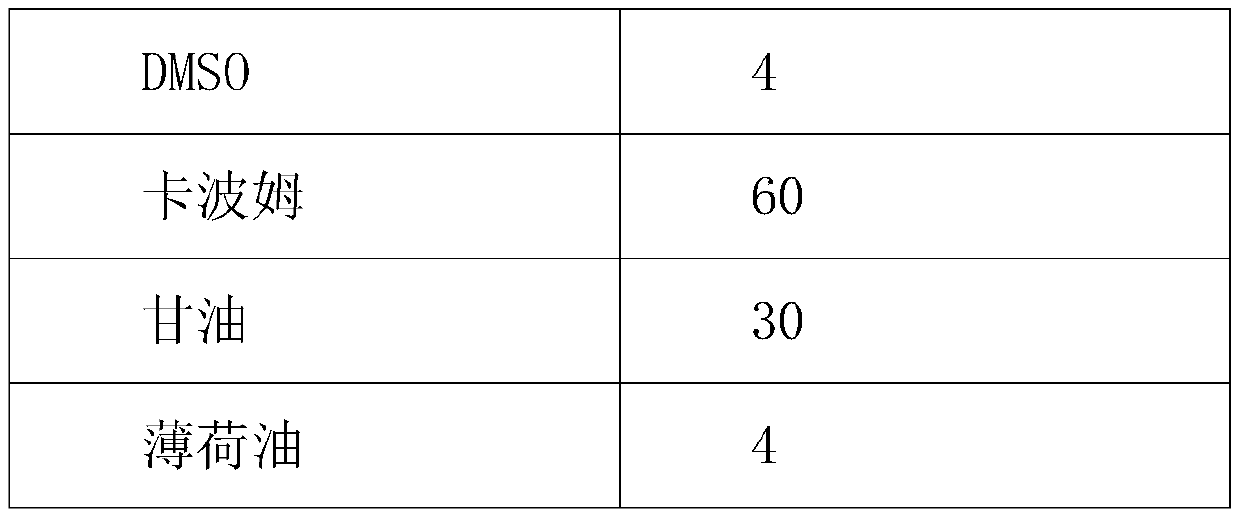
[0044] Table 3 oral gel composition 1
[0045] prescription composition Proportion in prescription (%) Apster 0.5 DMSO 2 Poloxamer 70 Triethanolamine 3 glycerin 20 peppermint 4.5
[0046] Preparation Process:
[0047] a) Dissolve the poloxamer in water to make it fully swell, then adjust the pH to neutral with a pH regulator, add glycerin and grind it sufficiently to make it moist;
[0048] b) After dissolving Apremilast in DMSO, slowly add it to a), grind while adding, and add an appropriate amount of distilled water to make it a gel with a suitable viscosity;
[0049] c) Add peppermint oil and triethanolamine to b), and grind to make it look like a transparent gel with a fragrant smell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com