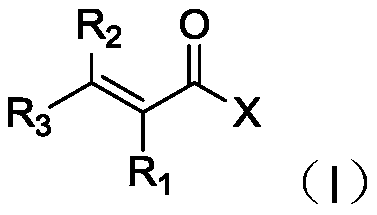
Method for selectively hydrogenating alpha, beta-unsaturated carbonyl compound by cobalt complex
A carbonyl compound and unsaturated technology, applied in the field of selective hydrogenation α of cobalt complexes, can solve the problems of high catalyst cost and high economic cost, and achieve the effects of high selectivity, low dosage and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 3-Phenylpropiophenone by Selective Hydrogenation of Chalcones
[0039]In the glove box, cobalt(II) acetylacetonate (26.0mg), 1,3-diisopropylimidazolium tetrafluoroborate (26.7mg), potassium carbonate (30.7mg) and ethanol (10.0g) were added to In a one-necked bottle equipped with a magnetic stirrer, start stirring, and after 20 minutes of dissolution and coordination of the metal precursor and ligand, a catalyst solution is obtained. Seal the one-necked bottle, take it out of the glove box, and protect it with a nitrogen balloon for later use. The autoclave was sealed, and there was no problem in the pressure-holding leak inspection, and the reactor was replaced 3 times with nitrogen. Feeding, first add the ethanol solution (10.0g) of the activator cuprous chloride (20.0mg) to the reaction kettle with an advection pump, then add the prepared catalyst solution before adding, finally add the solvent ethanol (45.0g), the substrate char Ketone (21.251 g). Afte...
Embodiment 2
[0041] Synthesis of 3-Phenylpropiophenone by Selective Hydrogenation of Chalcones
[0042] In the glove box, cobalt(II) acetylacetonate (2.6mg), 1,3-diisopropylimidazolium tetrafluoroborate (2.7mg), potassium carbonate (3.1mg) and ethanol (10.0g) were added to In a one-necked bottle equipped with a magnetic stirrer, start stirring, and after 20 minutes of dissolution and coordination of the metal precursor and ligand, a catalyst solution is obtained. Seal the one-necked bottle, take it out of the glove box, and protect it with a nitrogen balloon for later use. The autoclave was sealed, and there was no problem in the pressure-holding leak inspection, and the reactor was replaced 3 times with nitrogen. Feeding, first add the ethanol solution (10.0g) of activator cuprous chloride (10.1mg) in the reactor with advection pump, then add the prepared catalyst solution before adding, finally add solvent ethanol (22.6g), substrate Char Ketone (21.251 g). After all the materials are a...
Embodiment 3
[0044] Synthesis of 3-Phenylpropiophenone by Selective Hydrogenation of Chalcones
[0045] In the glove box, cobalt(II) acetylacetonate (51.9mg), 1,3-di-tert-butylimidazolium tetrafluoroborate (60.0mg), potassium carbonate (61.4mg) and ethanol (10.0g) were added in sequence Put it into a one-necked bottle equipped with a magnetic stirrer, start stirring, and after the metal precursor and ligand are dissolved and coordinated for 20 minutes, a catalyst solution is obtained. Seal the one-necked bottle, take it out of the glove box, and protect it with a nitrogen balloon for later use. The autoclave was sealed, and there was no problem in the pressure-holding leak inspection, and the reactor was replaced 3 times with nitrogen. Feeding, first add the ethanol solution (10.0g) of the activator cuprous chloride (50.5mg) to the reaction kettle with an advection pump, then add the prepared catalyst solution before adding, finally add the solvent ethanol (85.0g), the substrate char Keto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com